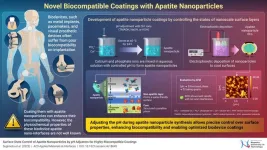
Medical implants have transformed healthcare, offering innovative solutions with advanced materials and technologies. However, many biomedical devices face challenges like insufficient cell adhesion, leading to inflammatory responses after their implantation in the body. Apatite coatings, particularly hydroxyapatite (HA)—a naturally occurring form of apatite found in bones, have been shown to promote better integration with surrounding tissues. However, the biocompatibility of artificially synthesized apatite nanoparticles often falls short of expectations, primarily due to the nanoparticles’ limited ability to bind effectively with biological tissues.
To overcome this challenge, researchers at Nagaoka University of Technology, Japan have developed a method for synthesizing surface-modified apatite nanoparticles that results in improved cell adhesion, offering new possibilities for the next generation of biocompatible medical implants. Led by Dr. Motohiro Tagaya, Associate Professor at the Department of Materials Science and Bioengineering at Nagaoka University of Technology, Japan, this research aims to enhance the performance of apatite coatings and advance the field of biocompatible materials for medical devices. The findings of this study were published online in ACS Applied Materials & Interfaces, on January 13, 2025, and in Volume 17, Issue 4 of the journal on January 29, 2025”. Along with Dr. Tagaya, Mr. Kazuto Sugimoto from Nagaoka University of Technology, Dr. Tania Guadalupe Peñaflor Galindo from Sophia University, and Mr. Ryota Akutsu from Nagaoka University of Technology were also a part of this research team.
Apatites are a class of calcium-phosphorus-based inorganic compounds, with hydroxyapatite—a naturally occurring form found in bones. These compounds are known for their high biocompatibility. Recent studies have found that coating artificial joints and implants with apatite nanoparticles is a plausible solution for improving the biocompatibility of these biodevices. However, the artificially synthesized nanoparticles often show reduced binding affinity to biological tissues in vitro. According to Dr. Tagaya and his team, this difference could be linked to the nanoscale surface layer of the apatite nanoparticles.
Dr. Tagaya’s research was driven by a desire to unravel the complexities of biocompatible materials, leading his team to develop an interdisciplinary framework that controls the intricate interactions between apatite and biological systems. “The properties of the nanoscale surface layer of apatite nanoparticles are crucial when considered for medical coatings,” adds Dr. Tagaya. Adding further, he says, “In this study, we successfully controlled the nanoscale surface layers of apatite nanoparticles, paving the way for advanced surface coating technologies for biodevices.”
The team synthesized hydroxyapatite nanoparticles by mixing aqueous solutions of calcium and phosphate ions. The pH of the solution was controlled using three different bases, which included tetramethylammonium hydroxide (TMAOH), sodium hydroxide (NaOH), and potassium hydroxide (KOH). The precipitated nanoparticles were then evaluated for their surface layer characteristics and were further used for coating via electrophoretic deposition.
The results revealed that pH was a key factor during synthesis, since it affected the crystalline phases, surface properties, and electrophoretic deposition. On analyzing the crystalline phases of the nanoparticles, it was observed that the choice of pH influenced the formation of different calcium phosphate phases like calcium-deficient hydroxyapatite (CDHA) and carbonate-containing hydroxyapatite (CHA). Higher pH favored the formation of CHA, leading to better crystallinity, and a higher calcium to phosphorus (Ca/P) molar ratio.
The surface of the apatite nanoparticles shows three different layers. The inner apatite layer/core is characterized by the presence of the crystalline structure of the apatite. Above the apatite layer is the non-apatitic layer, which is rich in ions like phosphate ions and carbonate ions. This layer reacts with water molecules and forms the hydration layer. Analyzing the surface characteristics of these layers revealed that pH adjustments facilitated the formation of the non-apatitic layer rich in reactive ions, enhancing hydration properties, which was confirmed.
Importantly, the study revealed that while higher pH facilitates the formation of the non-apatitic layer, the presence of Na+ ions reduces the concentration of phosphate ions, leading to decreased reactivity of the layer. The introduction of substantial ions by NaOH also affected the uniformity of electrophoretic deposition, as observed in scanning probe microscope studies. This effect was not observed with KOH, indicating that KOH was more suitable than NaOH for forming the non-apatitic layer and ensuring uniform coating.
Emphasizing the significance of the study, Dr. Tagaya says, “This study focuses on the critical interfaces between bioceramics and biological systems and could inspire designs of biocompatible surfaces with preferential cell adhesion.” These findings can be potentially useful for surface coating of a wide range of biodevices that are implanted in the human body, including artificial joints and implants.
Going ahead, the team intends to push the boundaries of nanobiomaterials, paving the way for groundbreaking innovations in medical materials and devices that could revolutionize healthcare and improve patient outcomes.
***
Reference
DOI: https://doi.org/10.1021/acsami.4c18645
About Nagaoka University of Technology, Japan
Nagaoka University of Technology (NUT), Japan, is a prominent university recognized for its excellence in engineering education and innovation. It is located in the city of Nagaoka, Japan. Established in 1976, the university fosters practical and creative engineers through its philosophy of "GIGAKU"—the science of technologies. NUT emphasizes the synergy between theory and practice and aims to contribute to global sustainability and drive technological innovation. Promoting its motto of VOS (Vitality, Originality, and Service), NUT reflects its commitment to advancing education and research for societal welfare. NUT is dedicated to cultivating leaders who integrate interdisciplinary knowledge to address real-world challenges while providing a culture of innovation, community, and diversity—driving research for a brighter future.
Website: https://www.nagaokaut.ac.jp/e/annai/intro/syokai.html
About Associate Professor Motohiro Tagaya from Nagaoka University of Technology, Japan
Dr. Motohiro Tagaya is currently an Associate Professor at the Nagaoka University of Technology, Japan. He earned his doctorate in engineering from the Tokyo Institute of Technology in 2010. A leading biomaterials researcher, he has over 175 publications and 2,294 citations. His work focuses on nanobioceramics, biomedical engineering, and designing interfaces for cellular therapeutics. Dr. Tagaya's contributions to bioceramics science and engineering have earned him the Inoue Research Award for Young Scientists of Japan in 2012. He continues to advance the field with innovative research, specializing in nanobiomaterials and bioceramics, driving impactful solutions in biomedical science.
Website: https://whs.nagaokaut.ac.jp/nanobio/
Funding information
This study was partially supported by a grant from the Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant-in-Aid for Challenging Exploratory Research, Grant 22K18916).
END