Fukuoka, Japan — Medical researchers in Japan have discovered a way to predict a potentially life-threatening side effect of cancer immunotherapy before it occurs. By analyzing cerebrospinal fluid collected pre-treatment, researchers at Kyushu University identified specific proteins associated with a damaging immune response that can affect the central nervous system after therapy. Their findings, published in Leukemia on 11 March, 2025, could make immunotherapy cancer treatment safer by helping doctors identify high-risk patients in advance, allowing early treatment or even prevention of this condition.
Over the past decade, cancer immunotherapy—where the patient’s immune system is used to fight cancer—has become a promising new treatment strategy. One form of immunotherapy, called CAR-T-cell therapy, uses genetic engineering to reprogram a patient’s immune cells (T cells) to target and destroy cancer cells. It has proven successful in treating blood cancers but comes with serious risks, including immune effector cell-associated neurotoxicity syndrome (ICANS), which causes inflammation in the central nervous system.
“ICANS can present with mild symptoms, such as headache or lethargy, but in more severe cases it can be life-threatening, with patients experiencing impaired consciousness, seizures or bleeding in the brain,” says Dr. Yuya Kunisaki, a professor in the Department of Clinical Chemistry and Laboratory Medicine, Kyushu University Hospital. “The incidence rate of ICANS after CAR-T therapy is very high, around 64%, but until now, there hasn’t been a reliable way to predict ICANS severity.”
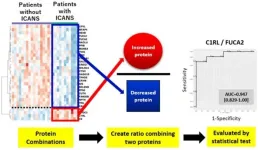
In this study, the research team analyzed the proteins in leftover cerebrospinal fluid taken from 29 patients with B-cell non-Hodgkin’s lymphoma before they received CAR T therapy. Within that cohort, 11 patients developed ICANS while 18 patients did not.
The research team identified 864 proteins present in all spinal fluid samples. From there, they narrowed the list down to 46 proteins that showed clear differences in levels between patients who developed ICANS and those who did not, making them potential biomarkers for predicting the condition.
Ultimately, the researchers found that C1RL, a protein with elevated levels in ICANS patients, and FUCA2, a protein with lower levels in ICANS patients, were the best predictors. When looking at these proteins together, a predictive test determined their ratio had high accuracy in distinguishing patients at high risk of ICANS from those at low risk.
The team then tested the C1RL/FUCA2 biomarker on a second group of 10 patients undergoing CAR-T therapy and found that for all patients, the protein ratio correctly determined the risk of developing ICANS.
However, the researchers cautioned that despite its high accuracy, the study’s small sample size means their findings are still preliminary. “We now need to conduct the study with a larger number of patients to fully validate our results,” says co-first author, Dr. Tomoko Nomiyama, a clinical technologist in the Department of Clinical Chemistry and Laboratory Medicine, Kyushu University Hospital.
In addition to helping doctors detect ICANS early and start treatment sooner, the researchers hope that identifying key biomarkers will allow doctors to give preventive drugs before CAR-T therapy begins, reducing the risk of ICANS developing at all. For example, C1RL, which is elevated in ICANS patients, is involved in the complement system—a part of the immune system that is known to cause inflammation and may contribute to ICANS.
“If the biomarker ratio shows a patient is at high risk for ICANS, we could preemptively treat them with drugs that inhibit the complement system to lower the risk,” explains Kunisaki. “This predictive test could therefore pave the way for a more personalized and safer approach to cancer treatment.”
The research team also plans to test if these biomarkers are accurate for patients with other blood cancers beyond B-cell non-Hodgkin’s lymphoma. Finally, they are also widening their search, hoping to uncover key biomarkers in more easily collected fluids, like blood serum.
“Spinal fluid collection is an invasive and painful procedure, so most hospitals in Japan and other countries don’t routinely perform it before CAR-T therapy,” says Nomiyama. “If we can identify similar biomarkers in blood, our test would become a much simpler and more accessible tool for predicting ICANS.”
###
For more information about this research, see “Cerebrospinal Fluid Proteomics Exerts Predictive Potential for ICANS in CAR-T Therapy.” Tomoko Nomiyama, Daiki Setoyama, Ikumi Yamanaka, Masatoshi Shimo, Kohta Miyawaki, Takuji Yamauchi, Fumiaki Jinnouchi, Teppei Sakoda, Kensuke Sasaki, Takahiro Shima, Yoshikane Kikushige, Yasuo Mori, Koichi Akashi, Koji Kato, Yuya Kunisaki, Leukemia, https://doi.org/10.1038/s41375-025-02541-6
About Kyushu University
Founded in 1911, Kyushu University is one of Japan's leading research-oriented institutes of higher education, consistently ranking as one of the top ten Japanese universities in the Times Higher Education World University Rankings and the QS World Rankings. The university is one of the seven national universities in Japan, located in Fukuoka, on the island of Kyushu—the most southwestern of Japan’s four main islands with a population and land size slightly larger than Belgium. Kyushu U’s multiple campuses—home to around 19,000 students and 8000 faculty and staff—are located around Fukuoka City, a coastal metropolis that is frequently ranked among the world's most livable cities and historically known as Japan's gateway to Asia. Through its VISION 2030, Kyushu U will “drive social change with integrative knowledge.” By fusing the spectrum of knowledge, from the humanities and arts to engineering and medical sciences, Kyushu U will strengthen its research in the key areas of decarbonization, medicine and health, and environment and food, to tackle society’s most pressing issues.
END