The Rice lab of chemist Andrew Barron revealed in a proof-of-concept study that amine-rich compounds are highly effective at capturing the greenhouse gas when combined with carbon-60 molecules.
The research is the subject of an open-access paper today in Nature's online journal Scientific Reports.
"We had two goals," Barron said. "One was to make the compound 100 percent selective between carbon dioxide and methane at any pressure and temperature. The other was to reduce the high temperature needed by other amine solutions to get the carbon dioxide back out again. We've been successful on both counts."
Tests from one to 50 atmospheric pressures showed the Rice compound captured a fifth of its weight in carbon dioxide but no measurable amount of methane, Barron said, and the material did not degrade over many absorption/desorption cycles.
Carbon-60, the soccer ball-shaped molecule also known as buckminsterfullerene (or the "buckyball") was discovered at Rice by Nobel Prize laureates Richard Smalley, Robert Curl and Harold Kroto in 1985. The ultimate curvature of buckyballs may make them the best possible way to bind amine molecules that capture carbon dioxide but allow desirable methane to pass through.
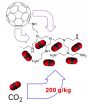
The Rice lab used buckyballs as crosslinkers between amines, nitrogen-based molecules drawn from polyethyleneimine. The lab produced a brown, spongy material in which hydrophobic (water-avoiding) buckyballs forced the hydrophilic (water-seeking) amines to the outside, where passing carbon dioxide could bind to the exposed nitrogen.
When Barron and his team began combining carbons and amines several years ago, they noticed an interesting progression: Flat graphene absorbed carbon dioxide well, multiwalled nanotubes absorbed it better, and thinner single-walled nanotubes even better. "That suggested the curvature was important," Barron said. "C-60, being a sphere, has the highest possible curvature among carbon materials."
He said the Rice compound compared favorably with other carbon-capture candidates based on metal organic frameworks (MOFs). "It's about equivalent to the best MOFs for carbon capture, but our material is far more selective. Methane just doesn't absorb," Barron said. Unlike MOFs, he noted the Rice compound absorbed wet carbon dioxide as well as dry.
Barron said it's just as important that the compound release carbon dioxide efficiently for reuse. "We noticed a long time ago that if we attached amines to carbon nanotubes or graphene, they lowered the temperature at which carbon dioxide dissolves," Barron said. Industrial amine-based scrubbers must be heated to 140 degrees Celsius to release captured carbon dioxide; lowering the temperature would save energy.
"Compared to the cost of current amine used, C-60 is pricy," Barron admitted. "But the energy costs would be lower because you'd need less to remove the carbon dioxide." He noted industrial scrubbers lose amines through heating, so they must constantly be replenished. "They're forever adding reagent, which is nice for the companies that sell amine, but not so good for those trying to separate the carbon dioxide."
The researchers are pursuing ways to improve the compound's capacity and rate of absorption. "We really understand the mechanism, which is important," Barron said. "That allows us to push it further."
INFORMATION:
Lead author Enrico Andreoli is a former Rice postdoctoral researcher and now a senior lecturer at Swansea University, Wales. Co-authors are former graduate student Eoghan Dillon, undergraduate alumna Laurie Cullum and senior research scientist Lawrence Alemany, all of Rice. Barron is the Charles W. Duncan Jr.-Welch Professor of Chemistry and a professor of materials science and nanoengineering.
The Apache Corp., the Robert A. Welch Foundation and the Welsh Government Ser Cymru Program supported the research.
David Ruth
713-348-6327
david@rice.edu
Mike Williams
713-348-6728
mikewilliams@rice.edu
Read the paper at http://www.nature.com/srep/2014/141203/srep07304/pdf/srep07304.pdf
This news release can be found online at http://news.rice.edu/2014/12/03/buckyballs-enhance-carbon-capture/
Follow Rice News and Media Relations via Twitter @RiceUNews
Related Materials:
Barron Research Group: http://barron.rice.edu/Barron.html
Rice Department of Materials Science and NanoEngineering: http://msne.rice.edu
Images for download:
http://news.rice.edu/wp-content/uploads/2014/12/1208_CARBON-1-WEB.jpg
Carbon-60 molecules, also known as buckyballs, were combined with amines in a compound that absorbs a fifth of its weight in carbon dioxide. It shows potential as an environmentally friendly material for capturing carbon from natural gas wells and industrial plants. (Courtesy of the Barron Research Group/Rice University)
http://news.rice.edu/wp-content/uploads/2014/12/1208_CARBON-2-WEB.jpg
Polyethyleneimine (PEI) with carbon-60 atoms, aka buckminsterfullerenes, form a spongy brown compound that absorbs a fifth of its weight in carbon dioxide but no measurable amount of methane. That may make it suitable for capturing carbon dioxide at wellheads and from industrial flue gases. (Courtesy of the Barron Research Group/Rice University)
http://news.rice.edu/wp-content/uploads/2014/12/1208_CARBON-3-WEB.jpg
Amines bound by buckyballs can absorb carbon dioxide from emissions at industrial plants and at natural gas wells, according to Rice University scientists who presented their work in Scientific Reports. (Courtesy of the Barron Research Group/Rice University)
http://news.rice.edu/wp-content/uploads/2014/12/1208_CARBON-4-WEB.jpg
Andrew Barron. (Credit: Jeff Fitlow/Rice University)
Located on a 300-acre forested campus in Houston, Rice University is consistently ranked among the nation's top 20 universities by U.S. News & World Report. Rice has highly respected schools of Architecture, Business, Continuing Studies, Engineering, Humanities, Music, Natural Sciences and Social Sciences and is home to the Baker Institute for Public Policy. With 3,920 undergraduates and 2,567 graduate students, Rice's undergraduate student-to-faculty ratio is just over 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice is highly ranked for best quality of life by the Princeton Review and for best value among private universities by Kiplinger's Personal Finance. To read "What they're saying about Rice," go here.