(Press-News.org) People who have had evidence of a prior infection with SARS-CoV-2, the virus that causes COVID-19, appear to be well protected against being reinfected with the virus, at least for a few months, according to a newly published study from the National Cancer Institute (NCI). This finding may explain why reinfection appears to be relatively rare, and it could have important public health implications, including decisions about returning to physical workplaces, school attendance, the prioritization of vaccine distribution, and other activities.
For the study, researchers at NCI, part of the National Institutes of Health, collaborated with two health care data analytics companies (HealthVerity and Aetion, Inc.) and five commercial laboratories. The findings were published on Feb. 24 in JAMA Internal Medicine.
"While cancer research and cancer care remain?the?primary?focus of NCI's work, we were eager to lend our expertise in serological sciences to help address the global COVID-19 pandemic, at the request of Congress," said NCI Director Norman E. "Ned" Sharpless, M.D., who was one of the coauthors on the study. "We hope that these results, in combination with those of other studies, will inform future public health efforts and help in setting policy."
"The data from this study suggest that people who have a positive result from a commercial antibody test appear to have substantial immunity to SARS-CoV-2, which means they may be at lower risk for future infection," said Lynne Penberthy, M.D., M.P.H., associate director of NCI's Surveillance Research Program, who led the study. "Additional research is needed to understand how long this protection lasts, who may have limited protection, and how patient characteristics, such as comorbid conditions, may impact protection. We are nevertheless encouraged by this early finding."
Antibody tests--also known as serology tests--detect serum antibodies, which are immune system proteins made in response to a specific foreign substance or infectious agent, such as SARS-CoV-2.
This study was launched in an effort to better understand whether, and to what degree, detectable antibodies against SARS-CoV-2 protect people from reinfection with the virus. Working with HealthVerity and Aetion, NCI aggregated and analyzed patient information collected from multiple sources, including five commercial labs (including Quest Diagnostics and Labcorp), electronic medical records, and private insurers. This was done in a way that protects the privacy of an individual's health information and is compliant with relevant patient privacy laws.
The researchers ultimately obtained antibody test results for more than 3 million people who had a SARS-CoV-2 antibody test between Jan. 1 and Aug. 23, 2020. This represented more than 50% of the commercial SARS-CoV-2 antibody tests conducted in the United States during that time. Nearly 12% of these tests were antibody positive; most of the remaining tests were negative, and less than 1% were inconclusive.
About 11% of the seropositive individuals and 9.5% of the seronegative individuals later received a nucleic acid amplification test (NAAT)--sometimes referred to as a PCR test--for SARS-CoV-2. The research team looked at what fraction of individuals in each group subsequently had a positive NAAT result, which may indicate a new infection. The study team reviewed NAAT results at several intervals: 0-30 days, 31-60 days, 61-90 days, and >90 days because some people who have recovered from a SARS-CoV-2 infection can still shed viral material (RNA) for up to three months (although they likely do not remain infectious during that entire period).
The team found that, during each interval, between 3% and 4% of the seronegative individuals had a positive NAAT test. But among those who had originally been seropositive, the NAAT test positivity rate declined over time. When the researchers looked at test results 90 or more days after the initial antibody test (when any coronavirus detected by NAAT is likely to reflect a new infection rather than continued virus shedding from the original infection), only about 0.3% of those who had been seropositive had a positive NAAT result--about one-tenth the rate in those who had been seronegative.
Although these results support the idea that having antibodies against SARS-CoV-2 is associated with protection from future infection, the authors note important limitations to this study. In particular, the findings come from a scientific interpretation of real-world data, which are subject to biases that may be better controlled for in a clinical trial. For example, it is not known why people who had tested antibody positive went on to have a PCR test. In addition, the duration of protection is unknown; studies with longer follow-up time are needed to determine if protection wanes over time.
To continue to comprehensively address this important research question, NCI is supporting clinical studies that monitor infection rates in large populations of people whose antibody status is known. These are known as "seroprotection" studies. NCI is also sponsoring ongoing studies using real-world data to assess the longer-term effect of antibody positivity on subsequent infection rates.
This research is part of a $306 million effort that NCI has taken on at the request of Congress to develop, validate, improve, and implement serological testing and associated technologies applicable to COVID-19. Through this appropriation, NCI is working with the Department of Health and Human Services; the National Institute of Allergy and Infectious Diseases, another part of NIH; and other government agencies to apply its expertise and advanced research capabilities to respond to this pandemic, including efforts to rigorously characterize the performance of serology assays.
INFORMATION:
About the National Cancer Institute (NCI): NCI leads the National Cancer Program and NIH's efforts to dramatically reduce the prevalence of cancer and improve the lives of cancer patients and their families, through research into prevention and cancer biology, the development of new interventions, and the training and mentoring of new researchers. For more information about cancer, please visit the NCI website at cancer.gov or call NCI's contact center, the Cancer Information Service, at 1-800-4-CANCER (1-800-422-6237).
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit nih.gov.
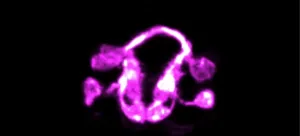
The formation of a brain is one of nature's most staggeringly complex accomplishments. The intricate intermingling of neurons and a labyrinth of connections also make it a particularly difficult feat for scientists to study.
Now, Yale researchers and collaborators have devised a strategy that allows them to see this previously impenetrable process unfold in a living animal -- the worm Caenorhabditis elegans, they report February 24 in the journal Nature.
"Before, we were able to study single cells, or small groups of cells, in the context of the living C. elegans, and for relatively short periods of time," said Mark Moyle, an associate research scientist in neuroscience at Yale School of Medicine and first author of the study. "It has been a breathtaking experience to ...
WOODS HOLE, Mass. -- Understanding how the brain works is a paramount goal of medical science. But with its billions of tightly packed, intermingled neurons, the human brain is dauntingly difficult to visualize and map, which can provide the route to therapies for long-intractable disorders.
In a major advance published next week in Nature, scientists for the first time report the structure of a fundamental type of tissue organization in brains, called neuropil, as well as the developmental pathways that lead to neuropil assembly in the roundworm C. elegans. This multidisciplinary study ...
What The Study Did: Medicare claims and clinical data were used to estimate health care costs associated with delirium in older adults one year after major elective surgery.
Authors: Tammy T. Hshieh, M.D., M.P.H., of Brigham and Women's Hospital in Boston, is the corresponding author.
To access the embargoed study: Visit our For The Media website at this link https://media.jamanetwork.com/
(doi:10.1001/jamasurg.2020.7260)
Editor's Note: The article includes conflicts of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, conflict of interest and financial disclosures, and funding and support.
INFORMATION:
Media advisory: The full study ...
What The Study Did: Researchers use a large set of clinical laboratory data linked to other clinical information such as claims to investigate the relationship between SARS-CoV-2 antibody status and subsequent nucleic acid amplification test (NAAT) results in an effort to understand how serostatus may predict risk of reinfection.
Authors: Lynne T. Penberthy, M.D., M.P.H., of the National Cancer Institute at the National Institutes of Health in Rockville, Maryland, is the corresponding author.
To access the embargoed study: Visit our For The Media website at this link https://media.jamanetwork.com/
(doi:10.1001/jamainternmed.2021.0366)
Editor's ...
In the last 60,000 years, humans have emerged as an ecologically dominant species and have successfully colonized every terrestrial habitat. Our evolutionary success has been facilitated by a heavy reliance on an ever-advancing technology. Understanding how human technology evolves is crucial to understanding why humans have enjoyed such unprecedented evolutionary success.
ASU doctoral graduate Jacob Harris, working with ASU researcher Robert Boyd and Brian Wood from the University of California Las Angeles and the Max Planck Institute for Evolutionary Anthropology, are interested in the role of causal ...
What The Study Did: This study combined the results of 12 studies with 1.8 million participants to examine the association between attention-deficit/hyperactivity disorder (ADHD) in childhood and adolescence and the subsequent risk of developing a psychotic disorder.
Authors: Mikaïl Nourredine, M.D., M.Sc., of the Hospices Civils de Lyon in Lyon, France, is the corresponding author.
To access the embargoed study: Visit our For The Media website at this link https://media.jamanetwork.com/
(10.1001/jamapsychiatry.2020.4799)
Editor's Note: The article includes conflict of interest disclosures. Please see the article for additional information, including other authors, author contributions and ...
BOSTON (February 24, 2021) - Results of a study published today in JAMA Surgery reveal the impact post-operative delirium has on health care costs in the U.S. Data from the study shows that if delirium were prevented or made less severe for patients, it could reduce health care costs by $33 billion per year, that is, $44,300 per patient per year. Severe delirium resulted in an additional $56,500 per patient per year, as compared to routine health care costs for older post-operative patients.
Tammy Hshieh, M.D., M.P.H., Adjunct Scientist, and Ray Yun Gou, M.A., Data Scientist II, both with the Aging Brain Center in the Hinda and Arthur Marcus Institute for Aging Research at Hebrew SeniorLife, are co-first authors. Sharon K. Inouye, M.D., M.P.H., Director ...
CHAPEL HILL, N.C.--For the last decade, the U.S. Food and Drug Administration has required tobacco manufacturers and importers to report the levels of harmful and potentially harmful chemicals found in their tobacco products and tobacco smoke. The idea was to educate the public and ultimately to decrease tobacco use, but little research has demonstrated if such information can impact on people's decisions to quit smoking.
A new study from the University of North Carolina at Chapel Hill has found that smokers who saw messages about tobacco chemicals with associated ...
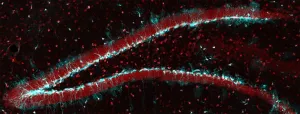
The stem cells in our brain generate new neurons throughout life, for example in the hippocampus. This region of the brain plays a key role for a range of memory processes. With increasing age, and in patients suffering from Alzheimer's disease, the hippocampus' ability to create new neurons declines steadily - and with it, its memory functions.
Distribution of age-dependent cell damage
A study conducted by the research group of Sebastian Jessberger, a professor at the Brain Research Institute of the University of Zurich, shows how the formation of new neurons is impaired with advancing age. Protein structures in the nuclei of neural stem cells make sure that harmful proteins accumulating ...
The most modern light sources for research are based on particle accelerators. These are large facilities in which electrons are accelerated to almost the speed of light, and then emit light pulses of a special character. In storage-ring-based synchrotron radiation sources, the electron bunches travel in the ring for billions of revolutions, then generate a rapid succession of very bright light pulses in the deflecting magnets. In contrast, the electron bunches in free-electron lasers (FELs) are accelerated linearly and then emit a single super-bright flash of ...