PORTLAND, Oregon -- New research from Oregon Health & Science University and collaborators indicates lab-made antibodies may be able to cure people infected with yellow fever, a virus for which there is no treatment.
The natural immune response to invading pathogens normally involves making protective proteins called antibodies. A study published today in Science Translational Medicine suggests that a single monoclonal antibody infusion can strengthen the body’s fight against yellow fever.
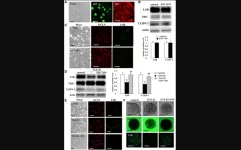
In the study, the yellow fever virus was undetectable in all animals that received monoclonal antibody infusions after being exposed to the virus.
“Two monoclonal antibodies that we evaluated completely removed all signs of infection from research animals,” said the study’s corresponding author, Ben Burwitz, Ph.D., associate professor at OHSU’s Vaccine and Gene Therapy Institute and affiliate associate professor at OHSU’s Oregon National Primate Research Center.
The collaborative research is a joint effort between scientists from OHSU, George Washington University, the biotechnology company Mabloc, LLC, and other organizations. Mabloc plans to use these findings to inform a future clinical trial in humans, in addition to their product development efforts.
“Neglected tropical diseases like yellow fever, dengue and Zika are often overlooked by traditional pharmaceutical development, but we hope monoclonal antibody technology will change that,” said David Watkins, Ph.D., co-senior author on the study, professor of pathology at George Washington University and Mabloc’s chief executive officer.
“By showing such efficacy in a primate model that mimics severe human disease, we hope to advance this to clinical trials and be ready to deploy treatments for the next outbreak of yellow fever,” said the study’s first author, Michael Ricciardi, Ph.D., associate director of translational research at George Washington University and Mabloc’s director of product development.
Increasingly common yellow fever
As many as half of people who get severe yellow fever die from the virus, which causes flu-like symptoms and can lead to jaundice and organ failure in more serious cases. Every year, the virus infects about 200,000 people — killing about 30,000 worldwide.
Currently, most cases occur in tropical and sub-tropical areas of Africa and South America, but global climate change is expected to increase the range of the mosquitoes that spread the virus. Deaths in Africa alone are predicted to increase by 25% by the year 2050.
The disease is preventable with a highly effective vaccine, which has been available since the 1930s. Although the vaccine is safe for the vast majority of people, vaccine hesitancy leaves some vulnerable to infection. It is a live vaccine that uses a weakened form of the virus, which causes a very small percentage of recipients to experience an adverse reaction that has been fatal in rare cases. A treatment could benefit both unvaccinated individuals who get sick and the very few people who experience a vaccine-related reaction.
When they began considering potential yellow fever treatments, the research team initially considered 37 antibodies cloned from people who had been vaccinated against yellow fever. The team then narrowed its focus to two monoclonal antibodies capable of controlling variants of the virus that were involved in recent yellow fever outbreaks.
The team manufactured these two monoclonal antibodies in the lab and studied how protective they could be against the yellow fever virus in two animal species: rhesus macaque monkeys and hamsters. After animals were exposed to the virus, each species was divided into three groups: one group that received the first antibody, another that received the second antibody, and a third that didn’t receive either antibody.
The virus couldn’t be detected in blood samples of any of the animals – eight rhesus macaques and 20 hamsters – that received either monoclonal antibody. All those in the control group developed severe disease. While one treated hamster died of an unknown cause, it neither showed signs of a yellow fever infection nor did it have signs of an adverse reaction to the monoclonal antibody.
Both monoclonal antibody candidates also appeared to be safe. None of the animals that received either experimental treatment displayed liver dysfunction, a tell-tale sign of severe yellow fever infection and yellow fever vaccine-associated disease.
This research was supported by the National Institutes of Health (grants R42 AI155275 and P51 OD01092) and by Mabloc, LLC.
Some of the researchers involved in this study -- including Jonah Sacha, Ph.D., of OHSU; and Michael Ricciardi, Ph.D., and David Watkins, Ph.D., of George Washington University -- have a significant financial interest in Mabloc, LLC, a company that may have a financial interest in the results of this research and technology. This potential individual and institutional conflict of interest has been reviewed and managed by OHSU.
All research involving animal subjects at OHSU must be reviewed and approved by the university’s Institutional Animal Care and Use Committee (IACUC). The IACUC’s priority is to ensure the health and safety of animal research subjects. The IACUC also reviews procedures to ensure the health and safety of the people who work with the animals. The IACUC conducts a rigorous review of all animal research proposals to ensure they demonstrate scientific value and justify the use of live animals.
REFERENCE: Michael J. Ricciardi, Lauren N. Rust, Nuria Pedreno-Llpez, Sofiya Yusova, Sreya Biswas, Gabriela M. Webb, Lucas Gonzalez-Nieto, Thomas B. Voigt, Johan J. Louw, Fernanda D. Laurino, John R. DiBello, Hans-Peter Raue, Aaron M. Barber-Axthelm, Kimberly Chun, Samantha Uttke, Lidiane M.S. Raphael, Aaron Yrizarry-Medina, Brandon C. Rosen, Rebeca Agnor, Lina Gao, Caralyn Labriola, Michael Axthelm, Jeremy Smedley, Justin G. Julander, Myrna C. Bonaldo, Laura M. Walker, Ilhem Messaoudi, Mark K. Slikfa, Dennis R. Burton, Esper G. Kallas, Jonah B. Sacha, David I. Watkins, Benjamin J. Burwitz, Therapeutic neutralizing monoclonal antibody administration protects against lethal yellow virus infection, Science Translational Medicine, March 29, 2023, https://doi.org/10.1126/scitranslmed.ade5795
Links:
Yellow Fever (CDC)
Benjamin Burwitz, Ph.D. (OHSU)
Related OHSU News stories:
New vaccine against tropical disease yellow fever to be tested (Sept. 30, 2019)
Hepatitis B cure sought with help of animal research (Oct. 13, 2020) END