(Press-News.org) DOWNLOADABLE VIDEO HERE
MIAMI, FLORIDA (EMBARGOED UNTIL SUNDAY, DEC. 10, 2023 AT 8:00 P.M. ET) – A study led by researchers at Sylvester Comprehensive Cancer Center at the University of Miami Miller School of Medicine suggests that CAR-T immunotherapy remains a viable option for patients who have lymphoma that goes into remission before the cell therapy begins.
While the study doesn’t answer the question of whether cell therapy in remission is the right choice, it does say that it’s not the wrong choice.
“I don’t think it answers the question of: Should we give these patients cell therapy? But I think it answers the question that we can – that it’s safe and that it’s a reasonable strategy when you’re in that spot,” said Trent Wang, D.O., a Sylvester hematologist and cellular therapy specialist who will present study findings in an oral presentation at the 65th ASH Annual Meeting and Exposition, the American Society of Hematology’s conference taking place in San Diego, California, Dec. 9-12.
Most patients receiving cell therapy, a form of immunotherapy that uses immune cells engineered to recognize and attack the patient’s cancer, desperately need it. For some, it comes after many other treatments have failed. But Wang noticed an odd phenomenon in the past few years when treating lymphoma patients with this form of therapy: Some of his patients went into complete remission before the cells ever touched their bodies.
This uncommon scenario occurs during the process of getting to cell therapy, which in the case of Wang’s study uses a kind of engineered immune cell known as CAR-T cells. When a patient starts the process, there’s a waiting period of three to five weeks before they get the treatment. Insurance approval is needed, and the cells themselves need to be manufactured from the patient’s own cells. But many of these patients are very sick with their cancer, so physicians will often treat them with a short course of chemotherapy or other drugs to tamp down the symptoms.
A small handful of these patients end up in remission during this waiting period treatment, the clinicians have found.
“That prompted this dilemma: Now what are we supposed to do?” Wang said. “Should we change the plan or give the therapy anyway? We just didn’t have a lot of information on this scenario.”
Wang said more often than not his team would proceed with the cell therapy in these cases, mainly to prevent yet another stretch of time where the patients’ cancer might come back again. But it didn’t feel like a very informed decision.
Wang and his colleagues noticed that their patients who received the cells while in remission tended to fare well after their infusion. But they didn’t know if those results would hold up in an analysis of a larger group. They proposed a research study to the Center for International Blood & Marrow Transplant Research, a nationwide registry that tracks patients who have received transplants and/or cell therapies.
The study included data from 134 patients in the registry who had gone into complete remission in the waiting period before receiving their cell therapy. To find that group, the scientists screened the records for more than 5,000 cell therapy patients.
They found that this group of patients had a 43% probability of progression-free survival over the two years following their treatment, about the same percentage as patients in the registry who were not in remission when they received CAR-T. However, the patients in remission had very low levels of toxicities related to their cell therapies, namely an immune overreaction known as cytokine release syndrome and neurotoxicity, two side effects that can sometimes accompany CAR-T cell therapy.
The study used data from patients treated with CAR-T cell therapy between 2015 to 2021, and current frequencies of specific cell therapy use are slightly different from those that were used in practice just a few years ago, Wang said. Next, the researchers want to explore the data paralleling more recent treatment trends.
Authors: Wang, first author, and Antonio M. Jimenez Jimenez, M.D., last author, are Sylvester researchers. Co-authors include Kwang Wooahn, Ph.D., Manmeet Kaur, and Mehdi Hamadani, M.D., Medical College of Wisconsin; Mazyar Shadman, M.D., Fred Hutchinson Cancer Center, Seattle; Alex F. Herrera, M.D., City of Hope, Duarte, California; and Craig S. Sauter, M.D., Taussig Cancer Institute, Cleveland.
Conflicts and disclosures: A full list of disclosures is included with the abstract.
# # #
Presentation Title: 615 Chimeric Antigen Receptor (CAR) T Cell Infusion for Large B Cell Lymphoma in Complete Remission: A Center for International Blood & Marrow Transplant Research (CIBMTR) Analysis
DOWNLOADABLE VIDEO HERE
END
SAN DIEGO ― Combining the JAK inhibitor ruxolitinib with the BCL-xL inhibitor navitoclax was twice as effective in reducing enlarged spleens – a major indicator of clinical improvement – compared with standard-of-care ruxolitinib monotherapy for adult patients with intermediate or high-risk myelofibrosis, a rare bone marrow cancer, according to results of the Phase III TRANSFORM-1 trial reported by researchers from The University of Texas MD Anderson Cancer Center.
Data from the global, randomized, placebo-controlled ...
SAN DIEGO ― Two clinical trials led by researchers from The University of Texas MD Anderson Cancer Center demonstrated early positive results from novel therapies targeting menin for the treatment of relapsed or refractory acute leukemias with specific genetic alterations. Results from the studies were shared today in oral presentations at the 2023 American Society of Hematology (ASH) Annual Meeting. More information on all ASH Annual Meeting content from MD Anderson can be found at MDAnderson.org/ASH.
Menin inhibitor monotherapy ...
SAN DIEGO ― The targeted therapy bezuclastinib was safe and rapidly reduced markers of disease burden while also improving symptoms for patients with a rare blood disorder called nonadvanced system mastocytosis, according to results of the Phase II SUMMIT trial reported by researchers at The University of Texas MD Anderson Cancer Center.
The findings, presented today at the 2023 American Society of Hematology (ASH) Annual Meeting, demonstrate that all participants treated with bezuclastinib achieved at least a 50% reduction in markers of disease burden and 63% reported their disease symptoms eased within 12 weeks. That number increased to 78% after ...
EMBARGOED UNTIL DECEMBER 9, 2023, AT 12:45 P.M. ET
A new study from researchers with Sylvester Comprehensive Cancer Center at the University of Miami Miller School of Medicine and collaborating organizations provides insight into the underlying mechanisms of gene mutations commonly seen in patients with myelodysplastic syndromes (MDS) and other myeloid neoplasms.
Their findings, to be presented at ASH 2023, the American Society of Hematology’s annual meeting in Santa Diego, Dec. 9-12, could lead to development of more effective drug combinations ...
SAN DIEGO – Early results from a Phase I clinical trial of AT101, a new CAR T cell therapy that uses a distinct binding mechanism to target CD19, show a 100 percent complete response (CR) rate at the higher dose levels studied in the trial, according to researchers from the University of Pennsylvania Perelman School of Medicine and Penn Medicine’s Abramson Cancer Center. The findings were published today in Molecular Cancer and presented at the 65th American Society of Hematology (ASH) Annual Meeting ...
Older patients with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL) who were not good candidates for the standard treatment of intensive chemotherapy had a median overall survival (OS) of 6.5 years on an alternate regimen of dasatinib and blinatumomab.
These long-term results from the S1318 clinical trial will be presented at the 65th American Society of Hematology (ASH) Annual Meeting and Exposition in San Diego on December 9 (abstract 1499).
Anjali S. Advani, MD, a SWOG investigator at Cleveland Clinic Cancer Institute, led the study, with ...
Mindfulness-Oriented Recovery Enhancement (MORE) — a behavioral intervention that integrates training in mindfulness, emotion regulation strategies and savoring of natural rewards — could hold the key to mitigating relapse in women undergoing medically assisted opioid use disorder treatment, a Rutgers study found.
The pilot study published in the journal Explore, is the first to evaluate the potential neural changes that underlie women’s emotion regulation and craving after an eight-week MORE intervention.
Previous studies have shown that women report higher opioid craving and show a greater inability to ...
Imagine if scientists developed a customizable cancer vaccine that was available — and affordable — for everyone. What if a patient scheduled for surgery also had the option of taking a pill whose composition includes nanorobotics capable of performing the procedure?
These and other medical scenarios may seem far-fetched and better suited to a science fiction thriller. However, the Advanced Research Projects Agency for Health (ARPA-H) is seeking to take such ideas from the drawing board to ...
Almost US$7 trillion per year in government subsidies and private investment - around 7 per cent of global GDP - has a direct negative impact on nature.
Nature-based solutions remain dramatically underfunded. Current public and private finance flows are only US$200 billion per year. To meet climate, biodiversity, and restoration targets, this needs to triple by 2030 and quadruple by 2050.
Realignment of public and private nature-negative finance flows is urgently needed
Dubai, 9 December 2022 – Close to $7 trillion is invested globally each year in activities that have a direct negative impact on nature from both public and private sector sources - equivalent to ...
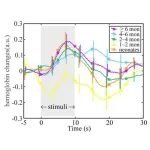
Tokyo, Japan – Researchers from Tokyo Metropolitan University have measured how oxygenated hemoglobin levels in the blood change in infants’ brains in response to touch. Using spectroscopy methods with external sensors placed on the scalp of sleeping infants, they found that the time at which levels peak doesn’t change with infant age, but the amount by which it varies over time does. Insights like this shed light on how the physiology of infants develop.
The first phase of a newborn’s life is a dazzling array of rapid developmental ...