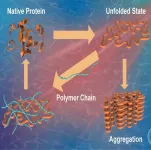
Ishikawa, Japan -- Proteins are vital biomolecules responsible for performing various functions in the human body and are thus regarded as the workhorses of a cell. The primary structure of a protein is composed of different amino acids coming together. The structure so formed then undergoes protein folding, a process by which a protein acquires its characteristic and functional three-dimensional configuration. This state, referred to as the ‘native state’, is crucial for proper protein function. Unfavorable conditions, such as stress or exposure to external agents, can cause proteins to misfold and form aggregates, hampering their ability to perform their original functions.
Protein misfolding has been implicated as the underlying cause of a range of human diseases, notably Alzheimer’s, Huntington’s, and Parkinson’s. Additionally, aggregate formation is also known to impact the efficacy and safety of protein-based drugs. This underscores the need for investigating compounds and strategies that can suppress misfolding and enhance protein stabilization.
Recent studies have reported the protein stabilization ability of a few polymers. However, their mechanism of action and the impact of interactions between hydrophobic components (the components that repel water) and proteins are not well understood.
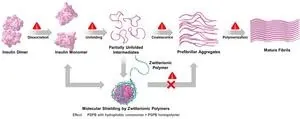
To address this knowledge gap, a team of researchers led by Professor Kazuaki Matsumura from the Japan Advanced Institute of Science and Technology (JAIST), including former Assistant Professor Robin Rajan, doctoral research fellow Dr. Dandan Zhao from JAIST, and Assistant Professor Tadaomi Furuta from the Tokyo Institute of Technology, conducted a study to elucidate the mechanism of protein aggregation inhibition by sulfobetaine (SPB). In their study published in Cell Reports Physical Science, the researchers also tried to understand the specific interactions that occur between hydrophobic components and proteins and their impact on protein aggregation.
Explaining the rationale behind this study, Prof. Matsumura says, "Previously, we conducted a study on polysulfobetaines (PSPBs), a zwitterionic polymer that consists of functional groups with both positive and negative charges. We found that the polymer showcased exceptional efficiency of in suppressing protein aggregation. However, the impact of hydrophobicity remained unexplored."
In this study, the researchers synthesized PSPBs with different molecular weights and added varying amounts of hydrophobic monomers individually and with different alkyl chains through a process known as reversible addition-fragmentation chain transfer polymerization. The researchers then analyzed the protein-stabilizing properties of these polymers and examined the interactions between polymers and proteins through physicochemical techniques.
Their findings revealed that offered protein stabilization by disrupting the important pathways involved in protein aggregation. Further, hydrophobicity and molecular weight both had an influence on preventing protein aggregation and enhancing protein stabilization. Increasing these factors amplified the weak and reversible interactions between SPB and proteins. “We can think of these polymers as reversible molecular shields, which disrupt the aggregation pathway," explains Prof. Matsumura, while discussing the results of their study. The researchers also found that on removal of stress, refolding of the partially unfolded intermediates was observed, suggesting regaining of their native states.
Thus, by unraveling the intricate molecular mechanisms of protein aggregation suppression by zwitterionic polymers, this pioneering study may open avenues for new therapeutic strategies that delay or prevent diseased conditions and help ensure the safety of protein-based drugs.
In the words of Prof. Matsumura, “In 5 to 10 years, this research could lead to the development of novel, more effective treatments for conditions related to protein misfolding, significantly improving patient outcomes. Additionally, it may enable the production of more stable and cost-effective protein therapeutics, benefiting the pharmaceutical industry and healthcare providers.”
###
Reference
Title of original paper:
Molecular mechanism of protein aggregation inhibition with sulfobetaine polymers and their hydrophobic derivatives
Authors:
Robin Rajan*, Tadaomi Furuta, Dandan Zhao, and Kazuaki Matsumura*
Journal:
Cell Reports Physical Science
DOI:
10.1016/j.xcrp.2024.102012
About Japan Advanced Institute of Science and Technology, Japan
Founded in 1990 in Ishikawa prefecture, the Japan Advanced Institute of Science and Technology (JAIST) was the first independent national graduate school in Japan. Now, after 30 years of steady progress, JAIST has become one of Japan’s top-ranking universities. JAIST counts with multiple satellite campuses and strives to foster capable leaders with a state-of-the-art education system where diversity is key; about 40% of its alumni are international students. The university has a unique style of graduate education based on a carefully designed coursework-oriented curriculum to ensure that its students have a solid foundation on which to carry out cutting-edge research. JAIST also works closely both with local and overseas communities by promoting industry–academia collaborative research.
About Professor Kazuaki Matsumura from Japan Advanced Institute of Science and Technology, Japan
Dr. Kazuaki Matsumura is currently a Professor at Japan Advanced Institute of Science and Technology (JAIST), Japan. He has published over 170 scientific articles in various fields of research, focusing mainly on biomaterials, biomedical engineering, and medical systems, and he has nearly 23 years of research experience. Dr. Matsumura is also the Director of the Research Center for Exponential Biomedical DX and the Director of Materials Chemistry Frontiers Research Area at JAIST. He received his Ph.D. in Engineering from Kyoto University in 2004.
Funding information:
This work was supported in part by a research grant Grant-in-Aid, KAKENHI (20K20197, 23K17211, 20H04532, and 21H05516), for scientific research from the Japan Society for the Promotion of Science and in part by the JST Adaptable and Seamless Technology Transfer Program through Target-driven R&D (A-STEP) (JPMJTR20UN).
END