Trey Pittman, a graduate student at Colorado State University, will present his team’s research at the fall meeting of the American Chemical Society (ACS). ACS Fall 2024 is a hybrid meeting being held virtually and in person Aug. 18-22; it features about 10,000 presentations on a range of science topics.
“Our device would be ideal for people who are at high risk for heart failure but have limited access to a hospital or a centralized lab,” says Pittman. “Working on this project to address health disparities in rural and low-resource areas really hits home for me because I’m from Mississippi, which has one of the highest mortality rates from heart failure in the United States,” he shares.
Heart failure refers to weakened heart muscle that cannot pump enough oxygenated blood through the body. The current gold standard for heart failure screening is a blood test administered twice per year by a health care professional that measures levels of B-type natriuretic peptide (BNP), a protein that indicates the heart is working too hard.
However, new developments in point-of-care devices may level the proverbial health care playing field with simple, at-home saliva tests. Such a test for heart failure screening could be administered by an individual to check on the health condition every few weeks instead of every six months, suggests Pittman. So far, widespread use of portable saliva tests for heart health has been limited by complicated manufacturing techniques and lack of relevant data beyond the presence or absence of a single biomarker.
Pittman and colleagues took on these challenges and have promising results to share about an intuitive, low-cost biosensor prototype, which they call an electrochemical capillary-driven immunoassay (eCaDI). Charles Henry’s group at Colorado State University combined two of their previous innovations to create the handheld testing platform: a saliva microfluidic device and a biosensor for biomarker proteins Galectin-3 and S100A7. Collaborator Chamindie Punyadeera’s group at Griffith University in Australia, quantified levels of Galectin-3 and S100A7 in saliva that correlated to heart failure outcomes.
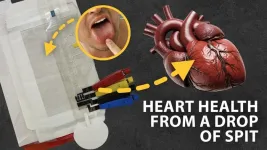
The heart failure eCaDI comprises five layers — like a club sandwich: Three layers of clear, flexible plastic held together with alternating layers of double-sided adhesive.
The top layer of plastic has tiny, drilled holes for loading the saliva sample. The middle plastic layer has laser-cut channels with squares of blotting paper at the end that draw saliva from the loading site through the channels. Wedged between the outer plastic layers are glass fiber reagent pads containing compounds that react with the saliva and measure Galectin-3 and S100A7 when an electrical current is applied to the device. The bottom layer of plastic has carbon ink electrodes screen printed on its surface. Two electrodes, powered by tiny, wired clamps from an external source called a potentiostat, drive the chemical reaction that happens on the reagent pads. “The devices are very easy to assemble,” says Pittman. “In about 20 to 30 minutes, we can make five of them.” The eCaDI is single use, and the researchers estimate that each one costs about $3.00. The potentiostat, a small reusable power source, sells for about $20.
In demonstrations, the researchers spiked standardized human saliva samples with levels of the two biomarkers that would indicate heart failure. Their results showed that the eCaDI accurately detected the amounts of Galectin-3 and S100A7 in the saliva. “These demos are a first step towards a robust and non-invasive electrochemical sensor for heart failure biomarkers,” says Pittman. In their next step, the team will test eCaDIs at Griffith University in human subject research trials with healthy individuals and those with heart failure.
“This work may provide a starting point for new saliva testing platforms for other diseases,” Pittman shares. “It’s a technology that I think could end up helping a lot of people — especially the underserved — live longer, healthier lives.”
The research was funded by the U.S. National Institutes of Health.
A Headline Science video about this topic will be posted on Monday, Aug. 19. Reporters can access the video during the embargo period, and once the embargo is lifted the same URL will allow the public to access the content. Visit the ACS Fall 2024 program to learn more about this presentation, “Development of a microfluidic electrochemical biosensor for heart failure biomarkers in saliva,” and other science presentations.
###
The American Chemical Society (ACS) is a nonprofit organization chartered by the U.S. Congress. ACS’ mission is to advance the broader chemistry enterprise and its practitioners for the benefit of Earth and all its people. The Society is a global leader in promoting excellence in science education and providing access to chemistry-related information and research through its multiple research solutions, peer-reviewed journals, scientific conferences, eBooks and weekly news periodical Chemical & Engineering News. ACS journals are among the most cited, most trusted and most read within the scientific literature; however, ACS itself does not conduct chemical research. As a leader in scientific information solutions, its CAS division partners with global innovators to accelerate breakthroughs by curating, connecting and analyzing the world’s scientific knowledge. ACS’ main offices are in Washington, D.C., and Columbus, Ohio.
Registered journalists can subscribe to the ACS journalist news portal on EurekAlert! to access embargoed and public science press releases. For media inquiries, contact newsroom@acs.org.
Note to journalists: Please report that this research was presented at a meeting of the American Chemical Society. ACS does not conduct research, but publishes and publicizes peer-reviewed scientific studies.
Follow us: X, formerly Twitter | Facebook | LinkedIn | Instagram
Title
Development of a microfluidic electrochemical biosensor for heart failure biomarkers in saliva
Abstract
Heart failure (HF) is a leading cause of death worldwide and continues to increase in prevalence as the general population ages. Advances in the healthcare management of heart failure have led to lower morbidity and mortality rates but require accurate diagnostics to guide the process. Current HF diagnostics require expensive equipment, centralized facilities and trained personnel and invasive sampling, marginalizing healthcare in developing countries and rural communities. New point-of-care (POC) diagnostic platforms that are portable, affordable, and use non-invasive samples can address this unmet need. Biosensors integrated into microfluidic platforms for biological sample processing offer many advantages for POC applications because they are inexpensive, portable, and easy-to-use. However, these platforms lack sensitivity, the ability to provide quantitative results, and the ability to process viscous biological samples. Electrochemical biosensors are sensitive, quantitative analytical methods popular in POC diagnostics due to the potential for inexpensive, portable instrumentation. An emerging non-invasive biological sample matrix that does not require trained personnel to collect is saliva. Recently, our team correlated concentrations of two salivary proteins (Galectin-3 and S100A7) to HF outcomes but no biosensors have been developed for measuring these biomarker levels at the POC. While saliva presents obstacles for processing in a microfluidic device, the development of a sensitive multiplexed POC biosensor for salivary biomarkers would improve healthcare management of HF. This work demonstrates an electrochemical multiplexed sandwich immunoassay based on a screen-printed carbon electrodes for the detecting the heart failure biomarkers, galectin-3 and S100A7 in pooled saliva samples. In parallel, a novel capillary driven microfluidic platform that can process varying viscosities of saliva in less than 15 minutes was developed. The proposed electrochemical immunoassay demonstrated a linear signal response in the clinically relevant range and a limit of detection of 9.66 ng/mL (galectin-3) and 39.0 ng/mL (S100A7). The preliminary results of the integration of the biosensor into the microfluidic platform indicate the first step towards a robust and non-invasive electrochemical sensor for HF biomarkers.
END