Hydrogen gas, owing to its high energy density and carbon-free nature, is gaining much attention as the energy source for a green and sustainable future. Despite being the most abundant element in the universe, hydrogen is mostly found in a bound state as chemical compounds such as ammonia, metal hydrides, and other hydrogenated compounds.
Among all the hydrogen carriers, ammonia stands out as a promising candidate owing to its wide availability, high hydrogen content with hydrogen making up 17.6% of its mass, and ease of liquefaction as well as transportation. A major drawback that hinders its exploitation as an on-demand green hydrogen source for practical applications is the need for extremely high temperatures (>773K) for its decomposition. Hydrogen production for fuel cells and internal combustion engine usage calls for high ammonia conversion rates at low temperatures.
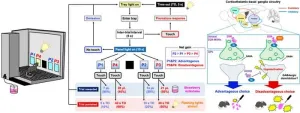
To solve this problem, a new compact process that could operate at a lower temperature was presented by Professor Yasushi Sekine from Waseda University, with his team including Yukino Ofuchi and Sae Doi from Waseda University, and Kenta Mitarai from Yanmar Holdings. They demonstrated an experimental setup of a high rate of ammonia-to-hydrogen conversion at remarkably lower temperatures by applying an electric field in the presence of a highly active and readily producible Ru/CeO2 catalyst. This study was published in Chemical Science on August 27, 2024.
“This is a collaborative project between our laboratory at Waseda University and Yanmar Holdings which is a leading company in ammonia utilization. We aimed to develop a process that would enable us to exploit the ability of ammonia to generate hydrogen on-demand,” states Sekine. Adding to this, he says, “So, we started investigating conventional thermal catalytic systems where the reaction proceeds through N and H adsorbate formation through dissociation of N–H bonds and the recombination of the adsorbates to form respective N2 and H2 gases,” while sharing the motivation behind the research study.
The team observed that the rate-determining step on an active metal Ru was the desorption of nitrogen at low temperatures and the dissociation of N–H at high temperatures. Their effort to overcome this issue led them to electric field-assisted catalytic reactions. This technique improved the proton conduction at the surface of the catalyst and reduced the activation energy required for the reaction along with its reaction temperatures to facilitate efficient ammonia conversion.
Using this information, the team designed a novel thermal catalytic system for low-temperature decomposition of ammonia to hydrogen assisted by easily producible Ru/CeO2 catalyst and DC electric field. They found that their proposed strategy efficiently decomposed ammonia even below 473 K. Given a long enough contact time between the ammonia feed and the catalyst, 100% conversion rate was achieved at 398 K, surpassing the equilibrium conversion rate. This was attributed to the electric field’s ability to promote surface protonics — proton hopping on the catalyst surface assisted by a DC electric field. This lowers the apparent activation energies of the ammonia conversion reaction.
In contrast, they observed that the lack of an electric field significantly slowed down the nitrogen desorption process causing the ammonia decomposition reaction to stop after some time. The significance of surface protonics in improving ammonia conversion rate was further supported by the experimental and density functional theory calculations carried out by the researchers.
This new strategy demonstrated that green hydrogen can be produced from ammonia at low temperatures with an irreversible pathway, ensuring almost 100% conversion at high reaction rates. “We believe that our proposed method can accelerate the widespread adoption of clean alternative fuels by making the on-demand synthesis of CO2-free hydrogen easier than ever,” concludes Sekine.
***
Reference
DOI: https://doi.org/10.1039/d4sc04790g
Authors: Yukino Ofuchia, Kenta Mitaraib, Sae Doia, Koki Saegusaa, Mio Hayashia, Hiroshi Sampeia, Takuma Higoa, Jeong Gil Seoc, and Yasushi Sekinea
Affiliations
aDepartment of Applied Chemistry, Waseda University, Tokyo, Japan
bResearch & Development Centre, Yanmar Holdings, Shiga, Japan
cDepartment of Chemical Engineering, Hanyang University, Seoul, Republic of Korea
About Waseda University
Located in the heart of Tokyo, Waseda University is a leading private research university that has long been dedicated to academic excellence, innovative research, and civic engagement at both the local and global levels since 1882. The University has produced many changemakers in its history, including nine prime ministers and many leaders in business, science and technology, literature, sports, and film. Waseda has strong collaborations with overseas research institutions and is committed to advancing cutting-edge research and developing leaders who can contribute to the resolution of complex, global social issues. The University has set a target of achieving a zero-carbon campus by 2032, in line with the Sustainable Development Goals (SDGs) adopted by the United Nations in 2015.
To learn more about Waseda University, visit https://www.waseda.jp/top/en
About Professor Yasushi Sekine
Yasushi Sekine is a Professor at the Department of Applied Chemistry at Waseda University. He received his bachelor’s and doctoral degree from The University of Tokyo. Prof. Sekine received a fellowship from the Royal Society of Chemistry for his research contributions and has been a fellow of Japan Science and Technology Agency since 2011. He has published over 230 research articles with more than 5700 citations. Currently, Sekine and his team are exploring catalysis at low temperatures using an electric field or reduction/oxidation cycle of lattice oxygen in oxide. His areas of expertise include green chemistry, chemical kinetics, and catalysis.
END