(Press-News.org) SAN FRANCISCO – Patients with metastatic colorectal cancer (mCRC) harboring BRAF V600E mutations benefitted from first-line treatment with the targeted therapies encorafenib and cetuximab plus a mFOLFOX6 chemotherapy regimen, according to results from the Phase III BREAKWATER trial led by researchers at The University of Texas MD Anderson Cancer Center.
The findings, presented today at the American Society of Clinical Oncology Gastrointestinal Cancers (ASCO GI) Annual Symposium and published in Nature Medicine, demonstrated a 60.9% overall response rate (ORR) with the three-drug combination compared to 40% with the standard-of-care (SOC) treatment – chemotherapy with or without bevacizumab. In the experimental arm, 68.7% of patients had a duration of response of at least six months, compared to 34.1% of patients in the SOC arm.
Data from this multi-institutional collaboration across 28 countries supported the accelerated approval of this combination by the Food and Drug Administration (FDA) in Dec. 2024, providing an effective new first-line treatment option for patients with BRAF V600E-mutant mCRC.
“Chemotherapy has had limited efficacy as a first-line treatment in controlling the aggressive tumor growth we see in patients with this mutation,” said co-principal investigator Scott Kopetz, M.D., Ph.D., professor of Gastrointestinal Medical Oncology and associate vice president of Translational Integration at MD Anderson. “This new regimen highlights the importance of combining dual-targeted therapy with chemotherapy to improve patient outcomes in the first-line setting, and the durable responses are a significant development as we work to improve quality of life for these patients.”
More than 150,000 people are diagnosed with colorectal cancer each year, making it the fourth most common cancer in the U.S., according to the National Cancer Institute. BRAF mutations occur in approximately 8-12% of cases and are associated with aggressive tumor growth, low efficacy from SOC treatments and a poor prognosis, with a median overall survival less than 12 months. Previously, there were no first-line targeted therapies approved for patients with BRAF V600E-mutant mCRC.
The BREAKWATER trial was one of the first studies to utilize the FDA’s Project FrontRunner, an initiative to encourage the evaluation of therapies in earlier clinical settings for advanced cancers rather than after patients received numerous previous treatments.
The trial enrolled patients who were at least 16 years of age with previously untreated BRAF V600E-mutant mCRC. Patients were randomized equally to one of three treatment arms: SOC chemotherapy with or without bevacizumab; a dual combination of encorafenib plus cetuximab; or a triple combination of encorafenib, cetuximab and mFOLFOX6.
When researchers analyzed patient subgroups on the trial, the triple combination showed benefits across important groups, including patients with cancer spread to three or more organs and those with liver metastases.
“These results support this combination as a new first-line standard of care for patients with BRAF V600E-mutant metastatic colorectal cancer,” Kopetz said. “It also highlights the importance of swiftly identifying molecular subtypes of colorectal cancer at diagnosis to optimize treatment strategies for our patients."
The safety profile of this combination was consistent with the known safety profile of each respective drug. No new safety signals were identified. The most common adverse reactions included nausea, rash, fatigue, vomiting, abdominal pain, diarrhea and decreased appetite, all of which were reported in at least 25% of patients and were similar between arms.
Final calculations of progression-free survival and overall survival will be formally assessed in the next phase of the trial. Future analyses of this trial may shed light on predictive biomarkers for this combination therapy.
The study was sponsored by Pfizer Inc., and Kopetz disclosed consulting for Pfizer and receiving research funding from the company. A full list of collaborating authors and their disclosures can be found in the full paper here.
Read this press release in the MD Anderson Newsroom.
END
Combination of dual-targeted therapies and chemotherapy shows high response rates in BRAF-mutated metastatic colorectal cancer
Phase III trial results supported recent FDA approval for patients with BRAF V600E mutations
2025-01-25
ELSE PRESS RELEASES FROM THIS DATE:
Blood test could guide use of anti-inflammatory drug celecoxib to reduce risk of colon cancer recurrence
2025-01-25
Boston – A data analysis from a randomized clinical trial for stage 3 colon cancer patients by investigators at Dana-Farber Brigham Cancer Center found that patients with evidence of residual cancer in their blood after surgery to remove the cancer, may benefit from adding of celecoxib, to post surgery treatment. The analysis showed that patients with positive blood tests for circulating tumor DNA (ctDNA) had worse outcomes in general, but those who were treated with celecoxib, a non-steroidal anti-inflammatory drug, experienced significantly improved disease-free survival.
“This is one of the first studies to show that ctDNA status has predictive utility in terms of selecting ...
Blood test from Alliance trial guides use of anti-inflammatory drug to lower colon cancer recurrence risk
2025-01-25
The Alliance for Clinical Trials in Oncology today announced the results of a data analysis from a randomized phase III clinical trial involving patients with stage III colon cancer, which found that adding the drug celecoxib to treatment after surgery might help those who still have traces of cancer in their blood. The analysis showed that patients with signs of cancer in their blood measured by Signatera™, a circulating tumor DNA (ctDNA) test, tended to have worse outcomes. However, those who took celecoxib after surgery had a much better chance of staying cancer-free. These results are being presented in a late-breaking ...
New dyes pave way for better photothermal cancer treatment and diagnosis
2025-01-25
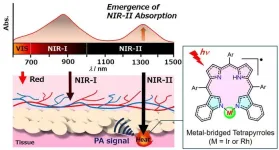
Tokyo, Japan – Researchers from Tokyo Metropolitan University have developed a new dye that can strongly absorb second near-IR radiation and transform it to heat. Starting with a dye from the bile pigment family, they designed a unique ring structure which can bind rhodium and iridium. Measurements and modeling revealed strong second near-IR absorptions and exceptional photostability. Second near-IR waves easily penetrate human tissue; the new dye may be applied in deep tissue therapies and imaging.
The second near-IR region of the electromagnetic spectrum (1000-1700 nanometers) ...
New drug shows promise in restoring vision for people with nerve damage
2025-01-25
Researchers at the University of Colorado Anschutz Medical Campus have found a promising drug candidate that could help restore vision in individuals with multiple sclerosis (MS) and other neurological conditions that damage neurons.
The study was published this week in the journal Nature Communications.
The drug, LL-341070, enhances the brain's ability to repair damaged myelin— the protective sheath around nerve fibers. Damage to myelin is a hallmark of diseases like MS, as well as a natural consequence of aging, often resulting in vision loss, loss of motor skills, ...
Scientists discover unique microbes in Amazonian peatlands that could influence climate change
2025-01-24
Complex organisms, thousands of times smaller than a grain of sand, can shape massive ecosystems and influence the fate of Earth's climate, according to a new study.
Researchers from Arizona State University, along with their colleagues from the National University of the Peruvian Amazon, have identified an unknown family of microbes uniquely adapted to the waterlogged, low-oxygen conditions of tropical peatlands in Peru’s northwestern Amazonian rainforest.
The new research shows these microbes have a dual role in the carbon cycle and the potential to either ...
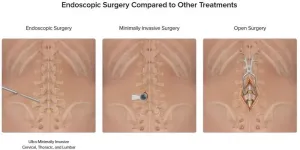
University Hospitals now offering ultra-minimally invasive endoscopic spine surgery for patients experiencing back pain
2025-01-24
CLEVELAND – University Hospitals is now offering endoscopic spine surgery for patients needing treatment for back pain due to herniated discs in their spine. Xiaofei (Sophie) Zhou, MD, completed Arthrex's Endoscopic Spine Training course to bring this advanced procedure to the health system and recently completed the first endoscopic discectomy utilizing Arthrex technology at UH. The health system is the only one in the greater Cleveland area offering this type of ultra-minimally invasive surgery.
Arthrex's technology allows surgeons to remove the ...
JNM publishes procedure standard/practice guideline for fibroblast activation protein PET
2025-01-24
Reston, VA (January 24, 2025)—The Society of Nuclear Medicine and Molecular Imaging (SNMMI) and the European Association of Nuclear Medicine (EANM) have issued a new procedure standard/practice guideline for the use of fibroblast activation protein (FAP) PET. Published in the January issue of The Journal of Nuclear Medicine, the procedure standard/practice guideline was developed to assist providers in recommending and performing FAP PET, as well as interpreting and reporting results of the imaging studies.
FAP is a transmembrane protein expressed on both cancer-associated fibroblasts and on normal activated fibroblasts involved in wound healing and ...
What to do with aging solar panels?
2025-01-24
The National Science Foundation Convergence Accelerator Program has granted $5 million dollars to Phase 2 of the project “Securing critical material supply chains by enabling phOtovoltaic circuLARity (SOLAR).”
SOLAR’s goal is to proactively ensure circularity of solar panels by providing solutions to barriers throughout the end-to-end supply chain. The intent is to make solar panels recyclable and find a solution to remanufacturing them at a competitive cost. Achieving this will help promote a clean and resilient energy system in the United States.
The three-year project is led by Battelle Memorial Institute ...
Scientists design peptides to enhance drug efficacy
2025-01-24
NEW YORK, NY, January 24, 2025 — A team of scientists has developed a groundbreaking approach using specially designed peptides to improve drug formulations. This innovative method significantly enhances anti-tumor efficacy, as demonstrated in leukemia models. The study, published in the journal Chem, was led by researchers at the Advanced Science Research Center at the CUNY Graduate Center (CUNY ASRC) and Memorial Sloan Kettering Cancer Center.
Drug delivery systems often face two critical challenges: poor solubility and inefficient delivery within the body. Many drugs do not dissolve well, making it difficult for them to reach ...
Collaboration to develop sorghum hybrids to reduce synthetic fertilizer use and farmer costs
2025-01-24
ST. LOUIS, MO, January 24, 2025 - A new collaborative research team of leading plant scientists are developing sorghums with nitrogen-saving traits by utilizing the genetic diversity of wild relatives to improve resilience and productivity for grain sorghum producers.
The project is part of a $38 million investment in nine projects by the U.S. Department of Energy, DOE, Advanced Research Projects Agency-Energy, ARPA-E, to develop advanced technologies for plants to increase nitrogen-use efficiency and reduce nitrogen pollution from U.S. bioenergy feedstocks.
Veena Veena, PhD, MBA, principal investigator and director of the Plant Transformation ...
LAST 30 PRESS RELEASES:
For solar power to truly provide affordable energy access, we need to deploy it better
Middle-aged men are most vulnerable to faster aging due to ‘forever chemicals’
Starving cancer: Nutrient deprivation effects on synovial sarcoma
Speaking from the heart: Study identifies key concerns of parenting with an early-onset cardiovascular condition
From the Late Bronze Age to today - Old Irish Goat carries 3,000 years of Irish history
Emerging class of antibiotics to tackle global tuberculosis crisis
Researchers create distortion-resistant energy materials to improve lithium-ion batteries
Scientists create the most detailed molecular map to date of the developing Down syndrome brain
Nutrient uptake gets to the root of roots
Aspirin not a quick fix for preventing bowel cancer
HPV vaccination provides “sustained protection” against cervical cancer
Many post-authorization studies fail to comply with public disclosure rules
GLP-1 drugs combined with healthy lifestyle habits linked with reduced cardiovascular risk among diabetes patients
Solved: New analysis of Apollo Moon samples finally settles debate about lunar magnetic field
University of Birmingham to host national computing center
Play nicely: Children who are not friends connect better through play when given a goal
Surviving the extreme temperatures of the climate crisis calls for a revolution in home and building design
The wild can be ‘death trap’ for rescued animals
New research: Nighttime road traffic noise stresses the heart and blood vessels
Meningococcal B vaccination does not reduce gonorrhoea, trial results show
AAO-HNSF awarded grant to advance age-friendly care in otolaryngology through national initiative
Eight years running: Newsweek names Mayo Clinic ‘World’s Best Hospital’
Coffee waste turned into clean air solution: researchers develop sustainable catalyst to remove toxic hydrogen sulfide
Scientists uncover how engineered biochar and microbes work together to boost plant-based cleanup of cadmium-polluted soils
Engineered biochar could unlock more effective and scalable solutions for soil and water pollution
Differing immune responses in infants may explain increased severity of RSV over SARS-CoV-2
The invisible hand of climate change: How extreme heat dictates who is born
Surprising culprit leads to chronic rejection of transplanted lungs, hearts
Study explains how ketogenic diets prevent seizures
New approach to qualifying nuclear reactor components rolling out this year
[Press-News.org] Combination of dual-targeted therapies and chemotherapy shows high response rates in BRAF-mutated metastatic colorectal cancerPhase III trial results supported recent FDA approval for patients with BRAF V600E mutations