(Press-News.org) UNDER STRICT EMBARGO UNTIL THURSDAY 27 MARCH 2025 AT 11 AM PACIFIC TIME / 2 PM EASTERN TIME .
Peer reviewed | Observational study | People
A new study from Queen Mary University of London found that 9% of all reported adverse drug reactions (ADRs) reported to the UK medicines regulator are associated with medications where side effect risk is partly dependent on patient’s genes. Of this subset of ADRs, 75% were associated with only three genes that impact how the body processes medication. Genetic testing before prescribing could therefore help avoid ADRs in these cases.
Over the past 60 years, The Medicines and Healthcare products Regulatory Agency (MHRA)’s Yellow Card scheme recorded over one million reports of side effects – also known as adverse drug reactions (ADRs) – to medications. Previous studies have indicated that more than 99% of individuals have genetic variants which could lead to an adverse response to certain drugs. In some cases, these reactions can be serious and lead to further health problems, longer hospital stays, or even death. The cost of ADRs to the NHS is estimated to be more than £2 billion a year.
A new study, published today in PLOS Medicine, led by Dr Emma Magavern from Queen Mary University of London analysed over 1.3 million ADR reports submitted to the MHRA Yellow Card scheme. It found that 115,789 (9%) were associated with drugs for which side effect risk can be modified using pharmacogenomics (PGx) information to guide prescribing. Of these, 75% were associated with three genes that affect the way an individual processes medication (CYP2C19, CYP2D6, SLCO1B1).
The type of medications that showed the highest volume of ADRs that could be prevented by personalising prescribing with genetic information were treatments for psychiatric disorders (47%) and cardiovascular problems (24%). The study also found that patients who had ADRs which were able to be mitigated by PGx were more likely to be male, older, and to experience side-effects that were severe but non-fatal.
Clinical trials have shown that using genetic information to guide prescribing pre-emptively, such as adjusting the dose or choosing different medication, can avoid ADRs and improve patient outcomes. This research highlights the potential of integrating pharmacogenomic testing into clinical practice to make medicines safer and more effective for patients.
Dr Emma Magavern, NIHR Clinical Academic Lecturer in Queen Mary’s Centre for Clinical Pharmacology and Precision Medicine who led the study, said: “It is important to understand the landscape of side effects reported nationally over the past half century to elucidate the impact that prospective use of genetic testing to personalise prescribing may have in the UK.”
Professor Sir Mark Caulfield, Vice-Principal (Health) at Queen Mary and co-author, said: “This is the largest analysis of the potential role of pharmacogenomics in adverse reactions from a national spontaneous reporting system. It suggests that 9% of these reports may relate to our genetic make-up. This could be avoidable if we had measured the genetic make-up of the person before prescribing these medicines. It is time for the NHS to consider adopting pre-emptive testing for known genes that interact with medications.”
June Raine, MHRA Chief Executive, said: “This study shows how reports of suspected side effects to the Yellow Card scheme can help us better understand and prevent serious side effects, including those linked to genetic factors. The MHRA Yellow Card scheme collects reports of suspected side effects from patients, the public and healthcare professionals and plays an important role in monitoring the safety of medicines in the UK. This research also reinforces the importance of our pioneering Yellow Card Biobank with Genomics England, which will help us take a more personalised, proactive approach to patient safety and make medicines safer for everyone.”
ENDS
NOTES TO EDITORS
The MHRA’s Yellow Card scheme is the UK’s system for collecting and monitoring reports of suspected side effects and adverse reactions to medicines and medical devices. It allows patients and healthcare professionals to flag potential safety concerns, improving medicine safety.
Contact:
Honey Lucas
Faculty Communications Officer – Medicine and Dentistry
Queen Mary University of London
Email: h.lucas@qmul.ac.uk or press@qmul.ac.uk
Paper details:
Emma F. Magavern et al. “Pharmacogenetics and adverse drug reports: insights from a United Kingdom national pharmacovigilance database.” Published in PLOS Medicine.
DOI: 10.1371/journal.pmed.1004565
Available after publication at: https://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1004565
Under strict embargo until Thursday 27 March 2025 at 11 AM PACIFIC TIME / 2 PM EASTERN TIME.
A copy of the paper is available upon request.
Conflicts of interest: The authors declare no conflicting interests.
Funded by: Dr Emma F. Magavern was funded by Barts Charity and an NIHR Clinical Lectureship. Maia Megase was funded by the University of Glasgow’s Life Sciences Vacation Scholarship. This work forms part of the funded research portfolio of the NIHR Barts Biomedical Research Centre.
About Queen Mary
www.qmul.ac.uk
At Queen Mary University of London, we believe that a diversity of ideas helps us achieve the previously unthinkable.
Throughout our history, we’ve fostered social justice and improved lives through academic excellence. And we continue to live and breathe this spirit today, not because it’s simply ‘the right thing to do’ but for what it helps us achieve and the intellectual brilliance it delivers.
Our reformer heritage informs our conviction that great ideas can and should come from anywhere. It’s an approach that has brought results across the globe, from the communities of east London to the favelas of Rio de Janeiro.
We continue to embrace diversity of thought and opinion in everything we do, in the belief that when views collide, disciplines interact, and perspectives intersect, truly original thought takes form.
About the MHRA
The Medicines and Healthcare products Regulatory Agency (MHRA) is responsible for regulating all medicines and medical devices in the UK by ensuring they work and are acceptably safe. Its work is underpinned by robust and fact-based judgements to ensure that the benefits justify any risks. The MHRA is an executive agency of the Department of Health and Social Care.
END
Testing patients for just three genes could help prevent three quarters of avoidable side effects of certain medications
2025-03-27
ELSE PRESS RELEASES FROM THIS DATE:
A genetic tree as a movie: Moving beyond the still portrait of ancestry
2025-03-27
ANN ARBOR—University of Michigan researchers have developed a statistical method that can be used for such wide-ranging applications as tracing your ancestry, modeling disease spread and studying how animals spread through geographic regions.
One of the method's applications is to give a more complete sense of human ancestry, says Gideon Bradburd, U-M professor of ecology and evolutionary biology. For example, when you send your DNA off for a personalized ancestry report, the report you get back is only a very small view of your family tree pinned in a specific point ...
New material gives copper superalloy-like strength
2025-03-27
Researchers from the U.S. Army Research Laboratory (ARL) and Lehigh University have developed a groundbreaking nanostructured copper alloy that could redefine high-temperature materials for aerospace, defense and industrial applications.
Their findings, published in the journal Science, introduce a Cu-Ta-Li (Copper-Tantalum-Lithium) alloy with exceptional thermal stability and mechanical strength, making it one of the most resilient copper-based materials ever created.
“This is cutting-edge science, developing a new material ...
Park entrances may be hotspots for infective dog roundworm eggs
2025-03-27
In an analysis of soil samples from twelve parks in Dublin, Ireland, park entrances were more heavily contaminated with infective roundworm eggs than any other tested park location. Jason Keegan of Trinity College in Dublin, Ireland, and colleagues present these findings in the open-access journal PLOS Neglected Tropical Diseases.
Dogs and cats are often infected with parasitic roundworms in the Toxocara genus. Infected animals can release the roundworm eggs into the environment, and humans can become infected after accidental ingestion of the eggs. Many infected humans never experience symptoms, but some may experience mild or severe ...
Commercial fusion power plant closer to reality following research breakthrough
2025-03-27
Successfully harnessing the power of fusion energy could lead to cleaner and safer energy for all – and contribute substantially to combatting the climate crisis. Towards this goal, Type One Energy has published a comprehensive, self-consistent, and robust physics basis for a practical fusion pilot power plant.
This groundbreaking research is presented in a series of six peer-reviewed scientific papers in a special issue of the prestigious Journal of Plasma Physics (JPP), published by Cambridge University Press.
The articles serve as the foundation for the company’s first fusion power plant project, which Type One Energy is developing with the Tennessee ...
The Protein Society announces its 2024 Best Paper recipients
2025-03-27
FOR IMMEDIATE RELEASE
Contact:
John Kuriyan, Editor-in-Chief
Protein Science Journal
Raluca Cadar
The Protein Society
Phone: (844) 377-6834
E-mail: rcadar@proteinsociety.org
LOS ANGELES, CA – The Protein Society, the premier international society dedicated to supporting protein research, announces the winners of the 2024 Protein Science Best Paper Awards, published in its flagship journal, Protein Science. The recipients will be recognized and present their research at the 39th Annual Symposium of The Protein Society, June 26 – 29, 2025, in San Francisco, USA.
The ...
Bing Ren appointed Scientific Director and Chief Executive Officer of the New York Genome Center
2025-03-27
The New York Genome Center (NYGC) is pleased to announce the appointment of Bing Ren, PhD, as its new scientific director and chief executive officer. Dr. Ren will also join Columbia University as a professor in the Departments of Genetics and Development, Biochemistry and Molecular Biophysics, and Systems Biology and as the associate director of the Roy and Diana Vagelos Institute for Basic Biomedical Science within the Vagelos College of Physicians and Surgeons.
Dr. Ren is renowned for his pioneering research in genomics and epigenetics, with a focus on the regulatory processes that control gene expression. His work has advanced our understanding of how genetic ...
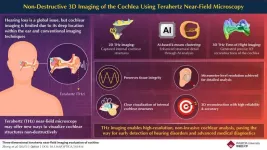
Terahertz imaging: a breakthrough in non-invasive cochlear visualization
2025-03-27
Advancements in healthcare and technology have significantly increased the average human lifespan. However, with longer life comes a higher prevalence of age-related disorders that affect overall well-being. One such condition is hearing loss in older adults, which can severely impact communication, social interactions, and daily functioning.
Hearing relies on the cochlea, a spiral-shaped organ in the inner ear that converts sound waves into neural signals. Any structural or functional impairment of the cochlea ...
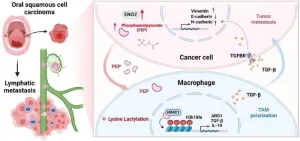
ENO2: a key player in head and neck squamous cell carcinoma metastasis
2025-03-27
A recent study published in Engineering has shed new light on the mechanisms underlying the metastasis of head and neck squamous cell carcinoma (HNSCC). The research identified enolase 2 (ENO2), a crucial glycolytic enzyme, as a significant factor associated with lymphatic metastasis in HNSCC.
HNSCC is an aggressive cancer with a relatively low 5-year overall survival rate. Cervical lymph node metastasis is a major cause of cancer-related death in HNSCC patients, and effective therapies for metastatic HNSCC are currently lacking. Therefore, understanding the molecular mechanisms of HNSCC metastasis ...
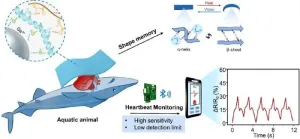
Biocompatible hydrogel enables wearable electronics for monitoring marine life health
2025-03-27
In a recent development published in Engineering, researchers have introduced a novel hybrid keratin (KE) hydrogel integrated with liquid metal (LM), offering new possibilities for monitoring the health of marine inhabitants. This innovation addresses the limitations of traditional wearable electronics in terms of biocompatibility, mechanical strength, and conductivity.
Monitoring the health and migration of marine organisms is crucial for maintaining the balance of marine ecosystems, advancing climate change studies, and safeguarding human health. However, developing sensors for marine organisms is challenging due to the complex ...
We must not ignore eugenics in our genetics curriculum, says professor
2025-03-27
To encourage scientists to speak up when people misuse science to serve political agendas, biology professor Mark Peifer of the University of North Carolina at Chapel Hill argues that eugenics should be included in college genetics curriculums. In an opinion paper publishing March 27 in the Cell Press journal Trends in Genetics, Peifer explains how he incorporated a discussion of eugenics into his molecular genetics course last year and why understanding the history of the field is critical for up-and-coming scientists.
“Eugenics is not dead but continues to influence science and policy today,” writes Peifer ...