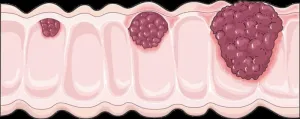
Colorectal cancer (CRC), a type of cancer that affects the large intestine and rectum, is one of the leading causes of cancer-related deaths worldwide. The mutational landscape of CRC is well characterized, revealing key pathogenic genetic abnormalities that drive carcinogenesis (cancer development) and disease progression. Moreover, a step-wise colorectal carcinogenesis model has been proposed wherein normal epithelial cells transition to adenoma (non-cancerous tumor) and then to carcinoma (cancerous tumor) as they sequentially acquire genetic mutations.
Mutations in APC, TP53, KRAS, and PIK3CA genes have been frequently reported in CRC patients and have been shown to drive tumor formation. However, the frequency of these mutations varies with the location of the tumor; APC and TP53 mutations are more frequent in left-sided colon cancer, whereas KRAS mutations are more frequent in right-sided colon cancer. Additionally, the location of the tumor also influences its morphology, immune cell filtration, prognosis, metastasis, and treatment response, suggesting that mechanisms underlying tumor development are likely site-specific.
Recently, BRAF mutations have been implicated in the development of tumors with a low frequency of APC, TP53, KRAS, and PIK3CA mutations. These tumors are known to develop via alternate genetic and epigenetic mechanisms, also known as the ‘serrated pathway.’ An alternate carcinogenesis model based on BRAF mutations, microsatellite instability (MSI), and CpG island methylator phenotype status has been proposed, although the underlying mechanisms remain unknown.
To bridge this gap, a team of researchers led by Dr. Hideyuki Saya, Director of the Oncology Innovation Center, Fujita Health University, Japan, including Associate Professor Tetsuya Takimoto, Dr. Yuko Chikaishi, and Dr. Hiroshi Matsuoka, analyzed CRC tumors with high tumor mutation burden (TMB) to characterize BRAF-associated mutations and decipher their role in the carcinogenesis of tumors lacking major driver oncogenes. Giving further insight into their work, in a study published in Cancer Science on January 16, 2025, Dr. Saya explains, “We observed that CRCs arising in the right and left colon differ in both their oncogenic mechanisms and biological characteristics. As a result, treatment approaches should also differ. Genome analysis for each cancer type can guide treatment selection and help improve the outcomes of patients with CRC.”
The researchers performed targeted exome sequencing—a gene sequencing technique used to analyze protein-coding regions of the genome using tumor samples obtained from 150 patients with CRC. They used a proprietary in-house cancer genome analysis system and assessed the type and frequencies of gene mutations based on TMB, MSI, and tumor site. 14 tumors were classified as TMB-high. Notably, 12 out of 14 tumors were located in the right colon and had a high BRAF mutation frequency and high MSI. Further, a high TMB was significantly associated with higher age and MSI-high status.
Additionally, mutations in DNA damage response transducers, such as ATM and POLE, and mismatch repair pathway genes MSH2 and MSH6, were frequent and significantly associated with a high TMB. Mutational signature analysis revealed that these mutations likely precede BRAF mutations associated with the activation of the serrated pathway, suggesting their potential role in early carcinogenesis.
While TMB-high tumors did not harbor APC, TP53, or KRAS mutations, the analysis revealed mutations in genes for pathways related to these key oncogenes, including mutations in receptor tyrosine kinase (RTK)-RAS pathway genes, BRAF, phosphatidylinositol 3-kinase (PI3K) pathway genes, PTEN, and NOTCH pathway genes; these mutations likely contribute to tumor survival and maintenance.
Overall, these findings suggest that TMB-high CRC tumors likely arise from a heterogeneous population of cells that harbors numerous gene mutations distinct from the driver oncogenes. The researchers thus speculate that these TMB-high tumors rely on alternate gene mutations which may respond well to targeted treatments and immunotherapies. Sharing his concluding thoughts, Dr. Saya says, “Currently, cancer genome analysis is performed only for a subset of cancer patients. However, in the future, it could well become a standard test for all cancers to better understand their genomic characteristics and devise appropriate treatment strategies.”
Currently, the team is optimizing the in-house cancer genome analysis system to integrate it into the diagnosis of CRC and tailor treatments based on genetic mutations. In the long term, these efforts could pave the way to several advancements in precision oncology.
***
Reference
DOI: 10.1111/cas.16455
About Fujita Health University
Fujita Health University (FHU) is a private medical university located in Aichi, Japan. Established in 1964, it houses one of the largest university hospitals in Japan. Its 900-member faculty provides diverse learning and research opportunities to medical students worldwide. Guided by its founding philosophy of "Our creativity for the people," FHU believes that its students can shape the future through creativity and innovation. FHU has earned global recognition, ranking eighth among all universities and second among private universities in Japan in the 2020 Times Higher Education (THE) World University Rankings. The university ranked fourth worldwide in the 2024 THE University Impact Rankings for contributions to the "Good Health and Well-being" Sustainable Development Goals (SDG) of the United Nations (UN). In June 2021, the university made history as the first Japanese institution to host the THE Asia Universities Summit. In 2024, FHU was awarded the Forming Japan’s Peak Research Universities (J-PEAKS) Program by the Japanese government to establish an innovative academic drug discovery ecosystem and hub of a multi-university consortium for research and education. Website: https://www.fujita-hu.ac.jp/en/index.html
About Dr. Hideyuki Saya from Fujita Health University
Dr. Hideyuki Saya is currently the Director of the Oncology Innovation Center at Fujita Health University. He obtained his Ph.D. from the Kobe University Graduate School of Medicine. Dr. Saya is dedicated to overcoming refractory cancers through fundamental research and its clinical applications. He aims to utilize his experience in basic cancer research and translational research to bridge the gap in clinical practice and to develop and practice early cancer diagnosis, groundbreaking treatment, and prevention based on emerging concepts.
Funding information
This study was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (KAKENHI 23H00410 to H.S.).
END