(Press-News.org) BOSTON -- A peer-reviewed paper published in The New England Journal of Medicine provides data from the much-anticipated COVE study, which evaluated mRNA-1273, a vaccine candidate against COVID-19 manufactured by Moderna, Inc. Results from the primary analysis of the study, which will continue for two years, provide evidence that the vaccine can prevent symptomatic infection. Among the more than 30,000 participants randomized to receive the vaccine or a placebo, 11 of those in the vaccine group developed symptomatic COVID-19 compared to 185 participants who received the placebo, demonstrating 94.1 percent efficacy in preventing symptomatic COVID-19. Cases of severe COVID-19 occurred only in participants who received the placebo.
Brigham and Women's Hospital served as a site for the trial as part of the COVID-19 Prevention Network (CoVPN), funded by the National Institutes of Health. In addition, Lindsey Baden, MD, an infectious diseases specialist at the Brigham and an expert in vaccine development for viral diseases, served as co-principal investigator for the study and lead author of the paper.
"Our work continues. Over the next months, we'll have increasing amounts of data to better define how this vaccine works, but the results so far show a 94.1 percent efficacy. These numbers are compelling," said Baden. "And, importantly, the data suggest protection from severe illness, indicating that the vaccine could have an impact on preventing hospitalizations and deaths, at least in the first several months post-vaccination."
The study enrolled 30,420 adult participants at 99 U.S. sites, including over 600 participants enrolled at the Brigham. Eligible participants were 18 years old or older with no known history of SARS-CoV-2 infection, and whose locations or circumstances put them at appreciable risk of SARS-CoV-2 infection and/or high risk of severe COVID-19. The race and ethnicity proportions of the trial were generally representative of U.S. demographics (79 percent white; 10 percent Black or African American; 20 percent Hispanic or Latino participants).
Participants received their first injection between July 27 and Oct. 23, 2020, followed by a second injection 28 days later. Each injection, given intramuscularly, had a volume of 0.5 mL, containing 100 μg of mRNA-1273 or saline placebo.
In the placebo group, 185 participants developed symptomatic COVID-19 illness; in the vaccine group, 11 participants did. In secondary analyses, the vaccine's efficacy was similar across groups of key interest, including those who already had antibodies against SARS-CoV-2 at the time of enrollment (indicating previous infection with COVID-19) and among those who were 65 years of age or older. Thirty participants had severe COVID-19 -- all in the placebo group.
Starting from randomization, cases of COVID-19 and severe COVID-19 were continuously monitored throughout the trial by the Data Safety Monitoring Board, empaneled by the NIAID. Participants were closely monitored for adverse events in the weeks following their injection. Investigators have collected and will continue to collect data on any serious adverse events or adverse events that require medical attention through two years post-injection.
Overall, reactions to the vaccine were mild -- about half of recipients experienced fatigue, muscle aches, joint pain and headaches, more so after the second dose. In most cases, these effects started about 15 hours after the vaccine and resolved after two days without sequelae. A similar number of adverse events were reported in the placebo and vaccine groups.
"While these results are encouraging, they are limited by the short duration of follow-up so far. Longer term data from the ongoing study may allow us to more carefully evaluate the vaccine's efficacy among different groups, determine the impact on asymptomatic infection, understand when immunity wanes, and determine whether vaccines affect infectiousness," said Baden. "But we shouldn't lose sight of the progress that we have made, which shows what is possible when we come together to tackle an immensely challenging problem."
INFORMATION:
This project has been funded in whole or in part with Federal funds from the Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority, under Contract No. 75A50120C00034. NIAID sources of grant funding included HVTN Leadership and Operations Center UM1 AI 68614HVTN Statistics and Data Management Center UM1 AI 68635, HVTN Laboratory Center UM1 AI 68618, HPTN Leadership and Operations Center UM1 AI 68619, ACTG Leadership and Operations Center UM1 AI 68636, and IDCRC leadership group 5 UM1 AI148684-03. Dr. Baden reports grants from NIH/NIAID during the conduct of the study. Dr. Baden is a Deputy Editor at the New England Journal of Medicine.
Paper cited: Baden L et al. "Efficacy and Safety of mRNA-1273 SARS-CoV-2 Vaccine" New England Journal of Medicine DOI: 10.1056/NEJMoa2035389
December 30, 2020 - As the COVID-19 pandemic emerged, trauma centers faced unprecedented obstacles to providing care for injured patients. A look at steps taken by trauma centers in response to COVID-19 is provided by a survey in the January/February Journal for Healthcare Quality (JHQ), the peer-reviewed journal of the National Association for Healthcare Quality (NAHQ). The journal is published in the Lippincott portfolio by Wolters Kluwer.
Trauma centers introduced new processes to optimize use of personal protective equipment (PPE), ICU beds, ventilators, and other limited resources, according to the report by David Bar-Or, MD, of ION Research, Englewood, Colo., ...
WHAT:
The investigational vaccine known as mRNA-1273 was 94.1% efficacious in preventing symptomatic coronavirus disease 2019 (COVID-19), according to preliminary results from a Phase 3 clinical trial reported in the New England Journal of Medicine. The vaccine also demonstrated efficacy in preventing severe COVID-19. Investigators identified no safety concerns and no evidence of vaccine-associated enhanced respiratory disease (VAERD).
The vaccine was co-developed by Moderna, Inc., a biotechnology company based in Cambridge, Massachusetts, and the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes ...
"Today's computer applications generate a large amount of data that needs to be processed by machine learning algorithms," says Yeseong Kim of Daegu Gyeongbuk Institute of Science and Technology (DGIST), who led the effort.
Powerful 'unsupervised' machine learning involves training an algorithm to recognize patterns in large datasets without providing labelled examples for comparison. One popular approach is a clustering algorithm, which groups similar data into different classes. These algorithms are used for a wide variety of data analyses, such as identifying fake news on social media, filtering spam in our e-mails, and detecting ...
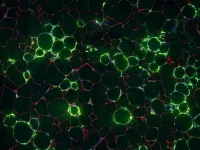
DALLAS - Dec. 30, 2020 - When fat cells in the body are stuffed with excess fat, the surrounding tissue becomes inflamed. That chronic, low-level inflammation is one of the driving factors behind many of the diseases associated with obesity. Now, UT Southwestern scientists have discovered a type of cell responsible, at least in mice, for triggering this inflammation in fat tissue. Their findings, published in Nature Metabolism, could eventually lead to new ways to treat obesity.
"The inflammation of fat cells in obese individuals is linked to many of the ...
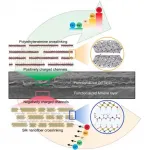
Lithium is an energy-critical element that is considered to be a geopolitically significant resource. However, the supply of lithium may not be enough to meet continuously increasing demand. As a result, scientists are looking for new ways to extract lithium ions.
Ion selective membranes have already been used extensively for water treatment and ion sieving in electrodialysis technology. However, conventional membranes exhibit low and useless Li+ selectivity, making them insufficient for meeting industry requirements.
Chinese scientists have recently made progress in the preparation and application of a bioinspired ...
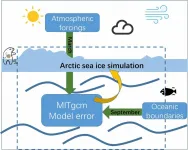
Arctic sea ice has been rapidly declining in recent decades, and changes in arctic sea ice can have a significant impact on global weather and climate through interactions with the atmosphere and oceans. In addition, the Arctic shipping routes are a shortcut to connect the major countries in the Northern Hemisphere. The Arctic region is also rich in natural resources and biological resources. Simulation of the Arctic sea ice could provide valuable information for Arctic shipping as well as climate studies, and it is therefore urgent to evaluate the ability to simulate Arctic sea ice and diagnose the sources of simulation errors.
To address the issue of error source identification, ...
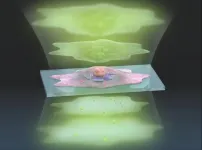
Experts in optical physics have developed a new way to see inside living cells in greater detail using existing microscopy technology and without needing to add stains or fluorescent dyes.
Since individual cells are almost translucent, microscope cameras must detect extremely subtle differences in the light passing through parts of the cell. Those differences are known as the phase of the light. Camera image sensors are limited by what amount of light phase difference they can detect, referred to as dynamic range.
"To see greater detail using the same image sensor, we must expand the dynamic range so that we can ...
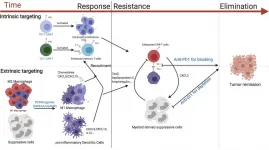
Activating an immune signaling pathway best known for fighting viral and bacterial infections can boost the ability of genetically engineered T cells to eradicate breast cancer in mice, according to a new study by researchers at the University of North Carolina. The study, to be published December 31 in the Journal of Experimental Medicine (JEM), suggests that CAR T cells, which are already used to treat certain blood cancers in humans, may also be successful against solid tumors if combined with other immunotherapeutic approaches.
Chimeric antigen receptor (CAR) T cells are a type of white blood cell that have been genetically engineered to recognize ...
The Asian tiger mosquito does not pose a major risk for Zika virus epidemics, according to a study published December 31 in the open-access journal PLOS Pathogens by Albin Fontaine of the Institut de Recherche Biomédicale des Armées, and colleagues.
Zika virus has triggered large outbreaks in human populations, in some cases causing congenital deformities, fetal loss, or neurological problems in adults. While the yellow fever mosquito Aedes aegypti is considered the primary vector of Zika ...
A novel computational drug screening strategy combined with lab experiments suggest that pralatrexate, a chemotherapy medication originally developed to treat lymphoma, could potentially be repurposed to treat Covid-19. Haiping Zhang of the Shenzhen Institutes of Advanced Technology in Shenzhen, China, and colleagues present these findings in the open-access journal PLOS Computational Biology.
With the Covid-19 pandemic causing illness and death worldwide, better treatments are urgently needed. One shortcut could be to repurpose existing drugs that were originally developed to ...