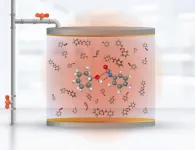
Controlling chemical reactions to generate new products is one of the biggest challenges in chemistry. Developments in this area impact industry, for example, by reducing the waste generated in the manufacture of construction materials or by improving the production of catalysts to accelerate chemical reactions.
For this reason, in the field of polariton chemistry - which uses tools of chemistry and quantum optics - in the last ten years different laboratories around the world have developed experiments in optical cavities to manipulate the chemical reactivity of molecules at room temperature, using electromagnetic fields. Some have succeeded in modifying chemical reactions products in organic compounds, but to date, and without relevant advances in the last two years, no research team has been able to come up with a general physical mechanism to describe the phenomenon and to reproduce it to obtain the same measurements in a consistent manner.
Now a team of researchers from Universidad de Santiago (Chile), part of the Millennium Institute for Research in Optics (MIRO), led by principal investigator Felipe Herrera, and the laboratory of the chemistry division of the US Naval Research Laboratory, (United States), led by researcher Blake Simpkins, for the first time reported the manipulation of the formation rate of urethane molecules in a solution contained inside an infrared cavity.
The discovery was published on June 16, 2023, in the chemistry section of the journal Science and proves, for the first time, both theoretically and experimentally, that it is possible to selectively modify the reactivity of certain bonds in a chemical reaction at room temperature in a liquid solvent, through the influence of the electromagnetic field vacuum in a narrow range of infrared frequencies. "This theoretical discovery improves our fundamental understanding of the phenomenon over other models that merely explain partial aspects of the experimental observations or simply refute the experimental evidence entirely," says researcher Felipe Herrera.
New scientific scope for molecule manipulation
Why is it so hard to control chemical reactions? When chemical reactions occur, the bonds that unite the atoms in a molecule brake and rearrange, forming new substances known as products. For this process to occur, energy is often needed, and several physicochemical principles dating from the nineteenth century have helped us understand how these energy transfer occurs according to the laws of thermodynamics.
There are also principles of reactivity based on the structures of molecules, such as those proposed by Eyring, Evans and Polanyi in 1935, widely used in all areas of chemistry. These basic principles imply that each reaction between two molecules is independent of the other chemical reactions that may occur in a solution. "That is very valid in almost all situations studied in eighty years and more, but the electromagnetic vacuum creates correlations between the different chemical reactions that happen within the volume of the cavity, and those correlations created by the electromagnetic field, in principle make the traditional assumptions of chemical reactivity questionable," explains Felipe Herrera.
"The experimental contribution of this study is the confirmation of the modification of the reaction rates through the interaction with the vacuum of the electromagnetic field confined inside the cavity, using a well-studied chemical reaction, and with more significant changes than those found with other types of reactions. In the theoretical part, the contribution is the fact that by modifying the dynamics of the chemical bonds that mainly participate in the reaction, through the infrared vacuum, it is possible to control the products", adds Johan Triana, a postdoctoral fellow at MIRO and the University of Santiago who participated in the creation of the mathematical model and the numerical calculations for the description of the molecular system.
Reproducing and interpreting measurements
The research started in 2020, when the then postdoctoral fellow at the US Naval Research Laboratory, now a professor at Bilkent University, Dr. Wonmi Ahn, performed the first experiments.
In 2021 Blake Simpkins prepared new samples to ensure that the measurements were reproducible and improved the liquid cells where the chemical reactions occur.
In the middle of that year, researcher Felipe Herrera began to have regular meetings with Simpkins to investigate possible theoretical answers to support the results obtained. "We decided to start from scratch and build a theory that takes all the physical aspects of quantum optics into consideration, but that under specific conditions reduces to the standard reactivity theory of theoretical chemistry," explains USACH professor Felipe Herrera.
The result of the process is the publication "Modification of ground-state chemical reactivity via light-matter coherence in infrared cavities", led by Simpkins (US Naval Research Laboratory) and Herrera (MIRO, Universidad de Santiago de Chile), with the participation of researcher Wonmi Ahn, (Bilkent University, Turkey), researcher Johan Triana and PhD student Felipe Recabal, both part of the Molecular Quantum Technology group of MIRO, at USACH.
This first work opens new possibilities and scientific challenges, Dr. Herrera explains: "We need to develop a sufficiently simple and general theoretical and mathematical framework that any researcher in the world can use to interpret their experiments and hopefully design new types of measurements that no one has yet visualized."
In this sense, Herrera reflects on his ambitions as a scientist moving across physics and chemistry: "It would be nice to build a consistent theory that unifies two of the most successful disciplines in modern science: chemical kinetics and quantum physics."
END