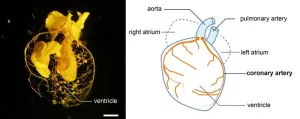
Coronary arteries are a vital part of the human heart, providing it with oxygen-rich blood so that it can work. By comparing the hearts of mammals, birds, reptiles, fish and frogs, a multi-institutional team of researchers appears to have found evidence that the structure of our hearts evolved in a stepwise process from fish, through amphibians to reptiles to mammals. When animals evolved from living in the water to living on land, a significant remodeling of the blood vessels to the heart occurred, enabling survival without gills. Understanding the ancestral origins of our cardiovascular system could help us find the basis for certain genetic defects. Knowing the systems of various other animals also broadens the scope for studying heart disease and potential treatments.
How do you take care of your heart? Being active, reducing stress and eating heart-healthy foods can all help to protect and strengthen this vital organ. Unfortunately, congenital heart disease (CHD) is the most common birth defect worldwide. CHD refers to a range of issues, including anomalous coronary artery, where the coronary arteries are malformed. These arteries supply the heart with oxygenated blood, so if they are not properly developed then it can cause sudden and dangerous complications. But not all species have this problem. There are animals including fish and amphibians whose hearts don’t have or need coronary arteries. So why do we and where did they come from?
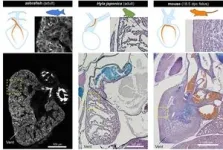
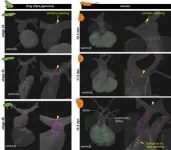
To help answer these questions, researchers in Japan compared the development of blood vessels in the hearts of mice, Japanese quails, newts, zebrafish, sharks and frogs. The team used a combination of techniques, including MRI, micro-CT scans, sectioning (analyzing thin slices of the hearts under a microscope) and 3D reconstruction, to create detailed images. By comparing these images, they could see the difference in how the blood vessels (vascular system) develop in the hearts of amniotes, i.e., mammals, birds and reptiles, and non-amniotes, i.e., fish, sharks and amphibians, from embryos to adults.
“In fish, sharks and amphibians, a long vascular system extending from the gills supplies blood to the heart. By contrast, in mammals and birds, this ancestral vasculature exists only during the embryo stage and later transforms into adult coronary arteries which are wrapped around the heart. This evolutionary change is likely connected to the transition of vertebrates from water to land, which led to the loss of gills,” explained researcher Hiroki Higashiyama from the Department of Physiological Chemistry and Metabolism at the Graduate School of Medicine at the University of Tokyo.
Amphibians sit in between fish and mammals on the evolutionary tree and so may offer some insight as to how one type of heart evolved into the other. “As an example, we saw that the transient blood vessels observed in mice embryos bore a striking resemblance to the ancestral vascular system found in amphibians,” said Higashiyama. “As the mouse's coronary arteries form, these ancestral vessels disappear, but they act as essential starting points for creating the new coronary arteries.”
The results appeared to show that the structure of the human heart originated from a common amniote ancestor. When this animal transitioned from being a water dweller to living on land, an important development took place which involved significant remodeling of ancestral blood vessels to form the coronary arteries we have today. The researchers speculated that this was necessary to enable a thickening of the human heart’s ventricle wall, so that it could handle high physiological activity, such as the elevated blood pressure that maintains amniotes’ high metabolic capacity (the ability to take on oxygen and circulate it in the blood).
“This research broadens the range of animal models available for studying heart diseases and their treatments,” said Higashiyama. “Next we would like to gain a deeper understanding of the molecular processes involved in coronary artery remodeling. By doing so, we can uncover the mechanisms of ‘how’ the mammalian- and reptilian-type coronary arteries evolved and potentially shed light on human congenital heart abnormalities and diseases.”
#####
Paper Title:
Kaoru Mizukami, Hiroki Higashiyama, Yuichiro Arima, Koji Ando, Norihiro Okada, Katsumi Kose, Shigehito Yamda, Jun K Takeuchi, Kazuko Koshiba-Takeuchi, Shigetomo Fukuhara, Sachiko Miyagawa-Tomita, Hiroki Kurihara. Coronary artery established through amniote evolution. eLife. doi: https://doi.org/10.7554/eLife.83005
Funding
This research was supported by the MEXT, Japan Society for the Promotion of Science (JSPS) Grant (20H04858 and 20K15858 (H.H); 22K07877 (S.M-T.); 19H01048 and 22H04991 (H.K.)), and Japan Science and Technology Agency (JST) Grant (PRESTO JP22715256 (Y.A.)).
Useful Links
Graduate School of Medicine: https://www.m.u-tokyo.ac.jp/english/
Research Contact:
Hiroki Higashiyama
Department of Physiological Chemistry and Metabolism
Graduate School of Medicine
The University of Tokyo
7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan
Email: h-hiroki@m.u-tokyo.ac.jp
Tel.: +81-3-5841-3496
Professor Hiroki Kurihara
Department of Physiological Chemistry and Metabolism
Graduate School of Medicine
The University of Tokyo
7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan
Email: kuri-tky@umin.net
Tel.: +81-3-5841-3496
Press contact:
Mrs. Nicola Burghall
Public Relations Group, The University of Tokyo,
7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8654, Japan
press-releases.adm@gs.mail.u-tokyo.ac.jp
About the University of Tokyo
The University of Tokyo is Japan's leading university and one of the world's top research universities. The vast research output of some 6,000 researchers is published in the world's top journals across the arts and sciences. Our vibrant student body of around 15,000 undergraduate and 15,000 graduate students includes over 4,000 international students. Find out more at www.u-tokyo.ac.jp/en/ or follow us on Twitter at @UTokyo_News_en.
END