(Press-News.org) For the first time, Stanford researchers have found a way to create and stabilize an extremely rare form of gold that has lost two negatively charged electrons, denoted Au2+. The material stabilizing this elusive version of the valued element is a halide perovskite—a class of crystalline materials that holds great promise for various applications including more-efficient solar cells, light sources, and electronics components.
Surprisingly, the Au2+ perovskite is also quick and simple to make using off-the-shelf ingredients at room temperature.
"It was a real surprise that we were able to synthesize a stable material containing Au2+—I didn't even believe it at first," said Hemamala Karunadasa, associate professor of chemistry at the Stanford School of Humanities and Sciences and senior author of the study published Aug. 28 in Nature Chemistry. "Creating this first-of-its-kind Au2+ perovskite is exciting. The gold atoms in the perovskite bear strong similarities to the copper atoms in high-temperature superconductors, and heavy atoms with unpaired electrons, like Au2+, show cool magnetic effects not seen in lighter atoms."
"Halide perovskites possess really attractive properties for many everyday applications, so we've been looking to expand this family of materials," said Kurt Lindquist, the lead author of the study who conducted the research as a Stanford doctoral student and is now a postdoctoral scholar in inorganic chemistry at Princeton University. "An unprecedented Au2+ perovskite could open some intriguing new avenues."
Heavy electrons in gold
As an elemental metal, gold has long been valued for its relative scarcity as well as its unmatched malleability and chemical inertness—meaning it can be easily shaped into jewelry and coins that do not react with chemicals in the environment and tarnish over time. An additional key reason for its value is gold's namesake color; arguably no other metal in its pure state has such a distinctively rich hue.
The fundamental physics behind gold's acclaimed appearance also explains why Au2+ is so rare, Karunadasa explained.
The root reason is relativistic effects, originally postulated in Albert Einstein's famed theory of relativity. "Einstein taught us that when objects move very fast and their velocity approaches a significant fraction of the speed of light, the objects get heavier,” Karunadasa said.
This phenomenon applies to particles, too, and has profound consequences for “massive” heavy elements, such as gold, whose atomic nuclei boast a large number of protons. These particles collectively exert immense positive charge, forcing negatively charged electrons to whirl around the nucleus at breakneck speeds. As a consequence, the electrons grow heavy and tightly surround the nucleus, blunting its charge and allowing outer electrons to drift farther than in typical metals. This rearrangement of electrons and their energy levels leads to gold absorbing blue light and therefore appearing yellow to our eye.
Because of the arrangement of gold's electrons, thanks to relativity, the atom naturally occurs as Au1+ and Au3+, losing one or three electrons, respectively, and spurning Au2+. (The “2+” indicates a net positive charge from the loss of two negatively charged electrons, and the "Au" chemical symbol for gold hails from “aurum,” the Latin word for gold.)
A squeeze of vitamin C
With just the right molecular configuration, Au2+ can endure, the Stanford researchers found. Lindquist said he "stumbled upon" the new Au2+-harboring perovskite while working on a broader project centered on magnetic semiconductors for use in electronic devices.
Lindquist mixed a salt called cesium chloride and Au3+-chloride together in water and added hydrochloric acid to the solution "with a little vitamin C thrown in," he said. In the ensuing reaction, vitamin C (an acid) donates a (negatively charged) electron to the common Au3+ forming Au2+. Intriguingly, Au2+ is stable in the solid perovskite but not in solution.
"In the lab, we can make this material using very simple ingredients in about five minutes at room temperature," said Lindquist. "We end up with a powder that's very dark green, nearly black, and is surprisingly heavy because of the gold it contains."
Recognizing that they may have hit new chemistry paydirt, so to speak, Lindquist performed numerous tests on the perovskite, including spectroscopy and X-ray diffraction, to investigate how it absorbs light and to characterize its crystal structure. Stanford research groups in physics and chemistry led by Young Lee, professor of applied physics and of photon science, and Edward Solomon, the Monroe E. Spaght Professor of Chemistry and professor of photon science, further contributed to studying the behavior of Au2+.
The experiments ultimately bore out the presence of Au2+ in a perovskite and, in the process, added a chapter to a century-old story of chemistry and physics involving Linus Pauling, who received the Nobel Prize in Chemistry in 1954 and the Nobel Peace Prize in 1962. Early in his career, he worked on gold perovskites containing the common forms Au1+ and Au3+. Coincidentally, Pauling also later studied the structure of vitamin C—one of the ingredients required to yield a stable perovskite containing the elusive Au2+.
"We love Linus Pauling’s connection to our work," Karunadasa said. "The synthesis of this perovskite makes for a good story."
Looking ahead, Karunadasa, Lindquist, and colleagues plan to study the new material further and tweak its chemistry. The hope is that an Au2+ perovskite can be used in applications that require magnetism and conductivity as electrons hop from Au2+ to Au3+ in the perovskite.
"We're excited to explore what an Au2+ perovskite could do," Karunadasa said.
Karunadasa is also a senior fellow at the Precourt Institute for Energy and a principal investigator and faculty scientist at the Stanford Institute for Materials and Energy Sciences, SLAC National Accelerator Laboratory. Solomon is a professor of photon science at Stanford Synchrotron Radiation Lightsource, SLAC. Additional Stanford co-authors are Christina R. Deschene and Alexander J. Heyer, graduate students in the Department of Chemistry; and Jiajia Wen, a staff scientist at SLAC. Additional co-authors include Armin Eghdami and Alexander G. Smith, graduate students in the Department of Physics, University of California-Berkeley, and Jeffrey B. Neaton, professor of physics at the University of California-Berkeley; and Dominic H. Ryan, professor of physics at McGill University.
The research was funded in part by the U.S. National Science Foundation, the U.S. Department of Energy, the Fonds de recherche du Québec–Nature et technologies, and the Natural Sciences and Engineering Research Council Canada.
END
Stanford researchers unveil new material infused with gold in an exotic chemical state
2023-09-29
ELSE PRESS RELEASES FROM THIS DATE:
Research Highlights for September 2023
2023-09-29
Huntsman Cancer Institute shines the spotlight on new discoveries and cutting-edge cancer research. This month, researchers found that increasing access for Black people with prostate cancer may save lives. Also, the first patient in a new small cell lung cancer clinical trial has been enrolled, researchers are using an app to help adolescents and young adults manage cancer symptoms, and investigators are trying to reduce cognitive side-effects after chemotherapy.
Increasing access to Black people with prostate cancer may decrease mortality rate
In a study published ...
JMIR Publications places No, 348 on The Globe and Mail's annual ranking of Canada's Top Growing Companies
2023-09-29
(Toronto, September 29, 2023) JMIR Publications is pleased to announce it placed No. 348 on the 2023 Report on Business ranking of Canada’s Top Growing Companies, making the ranking over the past three consecutive years.
Canada’s Top Growing Companies ranks Canadian companies on three-year revenue growth. JMIR Publications earned its spot with three-year growth of 105%.
“Being ranked on this list, year over year, showcases JMIR Publications’ national and global leadership in publishing high-quality open access ...
Argonne National Laboratory launches South Side STEM Opportunity Landscape Project at DuSable Black History Museum and Education Center
2023-09-29
A transformative initiative aimed at identifying, enhancing and promoting science, technology, engineering and mathematics resources within local communities.
The U.S. Department of Energy’s Argonne National Laboratory is proud to announce the official launch of the South Side STEM Opportunity Landscape Project, a transformative initiative aimed at identifying, enhancing and promoting science, technology, engineering and mathematics (STEM) resources within local communities.
As part of the Argonne in Chicago initiative that includes offices in Hyde Park, the ...
Allergy study on 'wild' mice challenges the hygiene hypothesis
2023-09-29
The notion that some level of microbial exposure might reduce our risk of developing allergies has arisen over the last few decades and has been termed the hygiene hypothesis. Now, an article published in Science Immunology by researchers from Karolinska Institutet challenges this hypothesis by showing that mice with high infectious exposures from birth have the same, if not an even greater ability to develop allergic immune responses than 'clean' laboratory mice.
How microbes may prevent allergy has been a topic of great interest in recent times. Studies have suggested that certain infections might reduce the production of inflammatory antibodies to ...
Ancient plant wax reveals how global warming affects methane in Arctic lakes
2023-09-29
New study makes novel use of plant biomarkers preserved in sediment to reconstruct methane cycling over the past 10,000 years
Plant waxes hold an isotopic signature of ancient methane
As planet warmed due to slow changes in Earth’s orbit, lakes produced increased amounts of methane, which is a potent greenhouse gas
Researcher: ‘Living on a warming planet, we can look to these signs from the past to predict our future’
EVANSTON, Ill. — By studying fossils from ancient aquatic plants, ...
Atopic dermatitis: Viruses discovered as new therapy option
2023-09-29
Up to 15 percent of children and five percent of adults are affected by the chronic inflammatory skin disease atopic dermatitis. Despite advanced therapy measures, the severe itching and eczema, especially on the elbows or knees, cause great distress to the patients. In the course of a study conducted at MedUni Wien a research team led by Wolfgang Weninger, Head of the Department of Dermatology, has discovered a new approach: bacteriophages, which colonize the skin as viral components of the microbiome and can drive the development of innovative ...
Larger lymph node threshold optimizes nasopharyngeal carcinoma outcomes
2023-09-29
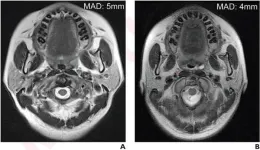
Leesburg, VA, September 29, 2023—According to the American Journal of Roentgenology (AJR), using a 6-mm threshold, rather than a 5-mm threshold, helps facilitate better risk stratification and treatment decisions in patients with nasopharyngeal carcinoma (NPC).
“Future American Joint Committee on Cancer (AJCC) staging updates should consider incorporation of the 6-mm threshold for N-category and tumor-stage determinations,” wrote corresponding author Zhiying Liang, MD, from the radiology department at China’s Sun Yat-sen University Cancer Center.
This AJR accepted manuscript by Liang et al. included ...
BPS celebrates Max Planck-Humboldt medal awardee Kandice Tanner
2023-09-29
ROCKVILLE, MD – The Biophysical Society is honored to celebrate Kandice Tanner, a physicist and Senior Investigator at the Center for Cancer Research, National Cancer Institute (NCI), National Institutes of Health (NIH) in Bethesda, Maryland. Tanner is being recognized for her pioneering work on the biophysics of the metastatic spread of cancer.
Using 3D organoid models of cancer progression, Tanner discovered a novel type of cell migration and cell generated forces associated with the formation of microtissues and tumors. This discovery demonstrated that physical forces are important in the establishment ...
Cleveland Clinic researchers develop new model for prioritizing lung transplant candidates
2023-09-29
September 29, 2023, CLEVELAND: A team from Cleveland Clinic has developed a new model for prioritizing patients waiting for a lung transplant, aimed at improving outcomes and reducing deaths among those in need of donor lungs. The new method offers an improved strategy for organ allocation by taking into account how the time a patient has spent on the waiting list could impact the severity of their disease and the urgency of their need for a transplant.
The results of a study looking at this new method were published today in The American Journal of Respiratory and Critical Care Medicine.
Currently, ...
American Academy of Arts and Sciences to induct UVA's Garcia-Blanco
2023-09-29
The University of Virginia School of Medicine’s Mariano A. Garcia-Blanco, MD, PhD, will be inducted this weekend into the American Academy of Arts and Sciences (AAAS), one of the country’s oldest and most prestigious honorary societies, in recognition of his exceptional scientific contributions.
The AAAS was founded in 1780 – during the Revolutionary War – by John Adams, John Hancock and other founding fathers who wanted to “cultivate every art and science which may tend to advance the interest, honor, dignity and ...