mRNA vaccines developed against the spike glycoprotein of severe acute respiratory syndrome type 2 coronavirus (SARS-CoV-2), displayed remarkable efficiency in combating coronavirus 19 (COVID-19). These vaccines work by triggering both cellular and humoral immune responses against the spike protein of the virus. Cellular immunity may play a more protective role than humoral immunity to variants of concerns (VOC) against SARS-CoV-2, as it targets the conserved regions of spike protein and possibly cross-reacts with other variants.
Since a single spike epitope is recognized by multiple T-cell clones, the mRNA vaccination-induced T-cell response may consist of multiple spike-reactive clones. Thus, it is important to understand the mechanism of mRNA vaccination-induced cellular immune response. However, to address this clonal-resolution analysis on T-cell responses to mRNA vaccination has not been performed yet.
To bridge this gap, a team of researchers, led by Associate Professor Satoshi Ueha, including Professor Kouji Matsushima from the Tokyo University of Science (TUS), Japan, Mr. Hiroyasu Aoki from the University of Tokyo, and Professor Toshihiro Ito from Nara Medical University, aimed to develop a kinetic profile of spike-reactive T-cell clones during repetitive mRNA vaccination. For this, they performed a longitudinal TCR sequencing on peripheral T cells of 38 participants who had received the Pfizer vaccine from before the vaccine to after the third vaccination and then analyzed the single-cell gene expression and epitope specificity of the clonotypes.
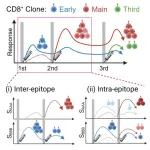
Their findings, published in Cell Reports on March 7, 2024, revealed that while the primary T-cell response of naïve T cells generally peaked 10-18 days after the first shot, expansion of "early responders" was detected on day 7 after the first shot, suggesting that these early responders contain memory T cells against common cold coronaviruses. They also found a "main responder" that expanded after the second shot and did not expand early after the first shot and a "third responder" that appeared and expanded only after the third shot.
By longitudinally tracking the total frequency of each response pattern, it was observed that, after the second shot, a shift among the clonotypes occurred, wherein the major population changed from early responders to main responders, suggestive of a shift in clonal dominance. A similar shift of responding clones was also observed in CD4+ T cells.
Expanding upon the research process, Prof. Ueha says, "We next analyzed the phenotype of main responders after the second and the third vaccination. The results showed that the main responders after the second and third shots mostly consist of effector-memory T cells (TEM), with more terminally differentiated effector memory-like phenotype after the third shot."
The researchers then examined the repertoire changes of main responders, revealing that the expansion of main responders, which occurred after the second shot, diminished following the third shot, and the clonal diversity decreased and was partially replaced by the third responders. This may potentially mean that the third vaccination selected better-responding clones.
Due to the vaccination-induced shift in immunodominance of spike epitopes, the study supports the inter-epitope shift model. In addition, there were intra-epitope shifts of vaccine-responding clonotypes within spike epitopes.
Prof. Ueha explains the significance of these results, “Our analysis suggests that T cells can “re-write” themselves and reshape their memory populations after successive vaccinations. This re-writability not only maintains the number of memory T cells but also maintains diversity that can respond to different variants of pathogens. Moreover, by tuning the replacement of memory cells, more effective vaccines can be developed that can also be tailored to an individual’s unique immune response.”
Overall, this study provides important insights into mRNA vaccine-induced T-cell responses, which will be crucial for developing next-generation vaccines for more effective and broad protection against viruses.
***
Reference
Title of original paper: CD8+ T-cell memory induced by successive SARS-CoV-2 mRNA vaccinations is characterized by shifts in clonal dominance
Journal: Cell Reports
DOI: https://doi.org/10.1016/j.celrep.2024.113887
Authors: Hiroyasu Aoki1,2, Masahiro Kitabatake3, Haruka Abe1, Peng Xu1, Mikiya Tsunoda1, Shigeyuki Shichino1, Atsushi Hara3, Noriko Ouji-Sageshima3, Chihiro Motozono4, Toshihiro Ito3, Kouji Matsushima1, and Satoshi Ueha1
Affiliations:
1Division of Molecular Regulation of Inflammatory and Immune Diseases, Research Institute for Biomedical Sciences, Tokyo University of Science
2Department of Preventive Medicine, Graduate School of Medicine, The University of Tokyo
3Department of Immunology, Nara Medical University
4Division of Infection and Immunity, Joint Research Center for Human Retrovirus Infection, Kumamoto University, Kumamoto
Further Information
Associate Professor Satoshi Ueha
Division of Molecular Regulation of Inflammatory and Immune Diseases
Research Institute for Biomedical Sciences
Tokyo University of Science
Email: ueha@rs.tus.ac.jp
Professor Toshihiro Ito
Department of Immunology,
Nara Medical University
Email: toshi-ito@naramed-u.ac.jp
Media contact
Hiroshi Matsuda
Public Relations Division
Tokyo University of Science
Email: mediaoffice@admin.tus.ac.jp
Website: https://www.tus.ac.jp/en/mediarelations/
Masaki Sakata
Research Support Division
Nara Medical University
Email: sangaku@naramed-u.ac.jp
Funding information
This work was supported by the Japan Society for the Promotion of Science (JSPS) under Grant Numbers 17929397, 20281832, 22H05064, and 23H02706, and Japan Agency for Medical Research and Development (AMED) under Grant Number JP22fk0310509, JP22fk0310514, JP22ama221306, and JP21gm6210025.
END