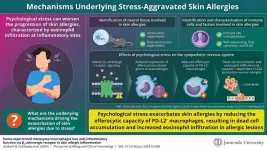
Psychological stress is known to exacerbate skin allergies, but the underlying molecular mechanisms remain poorly understood. Recent studies using a mouse model of immunoglobulin E (IgE)-mediated cutaneous allergic inflammation (IgE-CAI) suggest that stress may disrupt immune functions, thereby worsening allergic symptoms by interfering with the body's inflammatory responses. IgE-CAI is characterized by swelling and infiltration of eosinophils, a type of immune cell involved in allergic inflammation, at the affected site.
In a recent study, a research group led by Associate Professor Soichiro Yoshikawa, Professor Kenji Takamori, and Professor Sachiko Miyake from the Juntendo University Graduate School of Medicine, along with Dr. Hitoshi Urakami and Professor Shin Morizane from the Okayama University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences, explored the link between stress and allergic symptoms. Their study was published online on November 18, 2024, in The Journal of Allergy and Clinical Immunology.
The study revealed that psychological stress reduces the ability of specialized cells called anti-inflammatory programmed death ligand 2 (PD-L2)-positive macrophages, to clear dead cells at the allergy site, thereby exacerbating skin allergy symptoms. “This study is the first in the world to demonstrate that stress, through the sympathetic nervous system, disrupts macrophage function, which normally helps suppress allergic reactions, thereby intensifying allergic responses,” explains Dr. Yoshikawa.
To investigate the mechanism linking stress to skin allergies, the researchers used a mouse model, IgE-CAI, where injection of IgE caused persistent ear inflammation. The team first identified the neural tissue involved in IgE-CAI and then investigated the immune cells and factors contributing to the condition. They discovered that psychological stress was linked to a decrease in gene expression in macrophages responsible for clearing dead cells, a process known as efferocytosis. Additionally, they found that the accumulation of dead cells in the lesions led to increased infiltration of eosinophils, worsening the allergic response.
PD-L2-positive macrophages play a key role in maintaining anti-inflammatory functions by removing dead cells. However, the study reveals that psychological stress affects their function by disrupting the activity of the β2-adrenergic receptor (Adrb2) activity. Macrophages that mature under this altered Adrb2 signaling show a reduced ability to perform efferocytosis, leading to worsened skin allergies.
“Our findings suggest that the impact of psychological stress on immune cells is long-lasting and can even affect macrophages that differentiate later. This phenomenon, referred to as ‘stress memory,’ implies that severe stress leaves a lingering imprint on immune cells, influencing their function and contributing to disease development,” notes Dr. Yoshikawa.
The study further revealed that the accumulation of dead cells at the lesion site induced the expression of an eosinophil-recruiting protein, CCL24, contributing to the worsening of skin allergies. However, this expression was found to be dependent on caspase-1 enzyme activity. The researchers showed that administering a caspase-1 inhibitor reduced ear swelling caused by IgE-CAI and reversed eosinophil infiltration at the lesion site. These findings suggest that caspase-1 inhibitors and agents targeting CCL24 gene expression may be promising approaches for reducing skin allergies.
“Anti-inflammatory macrophages play crucial roles in various diseases, including cancer, autoimmune disorders, and wound healing. This study not only sheds light on the impact of stress on allergic inflammation but also lays the groundwork for exploring how stress exacerbates other diseases involving these macrophages,” explains Dr. Yoshikawa.
While avoiding stress altogether would be the ideal solution to prevent immune cell dysfunction, understanding the molecular mechanisms behind stress memory could pave the way for therapeutic strategies that alleviate or reverse these effects. Such advances could lead to novel treatments for stress-related diseases and have broad implications in clinical research.
Reference
Authors
Hitoshi Urakami¹,², Soichiro Yoshikawa¹,³,⁴, Kei Nagao¹,³,⁴, Kensuke Miyake⁵, Yuki Fujita¹,³, Ayaka Komura¹, Miho Nakashima¹, Ryusuke Umene⁶, Shuhei Sano³, Zheyu Hu3, Emi Nishii³, Atsushi Fujimura¹, Takeshi Y Hiyama⁷,⁸, Keiji Naruse¹,⁹, Hajime Karasuyama⁵, Tsuyoshi Inoue⁶, Mitsutoshi Tominaga⁴, Kenji Takamori⁴, Shin Morizane², and Sachiko Miyake³
Title of original paper
Stress-experienced monocytes/macrophages lose anti-inflammatory function via β2-adrenergic receptor in skin allergic inflammation
Journal
The Journal of Allergy and Clinical Immunology
DOI
10.1016/j.jaci.2024.10.038
Affiliations
1Department of Cellular Physiology, Okayama University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences, Japan
2Department of Dermatology, Okayama University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences, Japan
3Department of Immunology, School of Medicine, Juntendo University, Japan
4Juntendo Itch Research Center (JIRC), Institute for Environmental and Gender-Specific Medicine, Juntendo University Graduate School of Medicine, Japan
5Inflammation, Infection & Immunity Laboratory, Advanced Research Institute, Tokyo Medical and Dental University (TMDU), Japan
6Department of Physiology of Visceral Function and Body Fluid, Graduate School of Biomedical Sciences, Nagasaki University, Japan
7Department of Integrative Physiology, Tottori University Graduate School and Faculty of Medicine, Japan
8International Platform for Dryland Research and Education, Tottori University, Japan
9Department of Cardiovascular Physiology, Okayama University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences, Japan
About Associate Professor Soichiro Yoshikawa
Dr. Soichiro Yoshikawa is an Associate Professor at the Juntendo Itch Research Center (JIRC), Institute for Environmental and Gender-Specific Medicine, Juntendo University Graduate School of Medicine. He has made significant contributions to the fields of immunology, neuroimmunology, allergy pathophysiology, and cell signaling, with nearly 74 scientific publications to his credit. His research explores the mechanisms underlying immune responses and the pathophysiology of allergic conditions, offering valuable insights into cellular processes and signaling pathways involved in these areas.
END