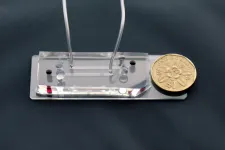
(Press-News.org) The novel label-free assay uses unconventional L and inverse-L shaped pillars of deterministic lateral displacement (DLD) microfluidic technology to quantify and profile immune states of white blood cells (WBCs) by assessing biophysical properties of size, deformation, distribution, and cell count
The assay requires only 20 microlitres (μl) of unprocessed blood and takes just 15 minutes - much faster than existing methods which require up to 15 millilitres (ml) of blood and take at least a few hours to produce results
This new technology measures and profiles the often volatile host immune response, resulting in a more accurate assessment of patient pathophysiology
Current methods for early diagnosis of infection focus on detecting low-abundance pathogens, and are time-consuming, of low sensitivity, and do not accurately reflect the severity of infection
Singapore, 25 March 2021 - Researchers from Critical Analytics for Manufacturing Personalized-Medicine (CAMP), an Interdisciplinary Research Group (IRG) at the Singapore-MIT Alliance for Research and Technology (SMART), MIT's research enterprise in Singapore, have developed a new label-free immune profiling assay that profiles the rapidly changing host immune response in case of infection, in a departure from existing methods that focus on detecting the pathogens themselves, which can often be at low levels within a host. This novel technology presents a host of advantages over current methods, being both much faster, more sensitive and accurate.
The new assay is described in a paper titled, "Label-free biophysical markers from whole blood microfluidic immune profiling reveals severe immune response signatures", published recently in Small, a weekly peer-reviewed scientific journal covering nanotechnology, and included a pilot study of 85 donors recruited from the National University Hospital (NUH) emergency department. The paper was led by Dr Kerwin Kwek Zeming, senior postdoctoral associate at SMART CAMP, and co-authored by Professor Jongyoon Han, Principal Investigator at SMART CAMP and Professor of Biological Engineering and Electrical Engineering at MIT, and Dr Win Sen Kuan, Research Director, Emergency Medicine Department, NUH.
In many cases, the main culprit behind disease manifestation, severity of infection, and patient mortality is an overly aggressive host immune response. For instance, the Spanish Flu pandemic of 1918 resulted in a disproportionately high number of deaths among otherwise healthy young adults. This has been attributed to the now well-studied phenomenon of cytokine storms, which precipitate the rapid release of immune cells and inflammatory molecules and are brought on by a hyper-aggressive host immune response. In a more recent example, cases of severe COVID-19 infection often result in death via sepsis and a dysregulated immune response, while current risk stratification methods based on age and comorbidity remain a significant challenge and can be inaccurate. Moreover, current Covid-19 testing does not prognose the severity of the immune response and can thus lead to inefficient deployment of resources in healthcare settings.
In cases of acute infection, the status of a patient's immune response can often be volatile and may change within minutes. Hence, there exists a pressing need for assays that are able to rapidly and accurately inform on the state of the immune system. This is particularly vital in early triage among patients with acute infection and prediction of subsequent deterioration of disease. In turn, this will better empower medical personnel to make more accurate initial assessments and deliver the appropriate medical response. This can ensure timely intervention in the emergency department (ED) and prevent admission to the intensive care unit (ICU).
The new assay developed by SMART researchers focuses on profiling the rapidly changing host inflammatory response, which in a hyper-aggressive state, can lead to sepsis and death. A 15-minute label-free immune profiling assay from 20 μL of unprocessed blood using unconventional L and inverse-L shaped pillars of DLD microfluidic technology was developed, functioning as a sensitive and quantitative assay of immune cell biophysical signatures in relation to real-time activation levels of WBCs. As WBCs are activated by various internal or external triggers, the assay can sensitively measure both the extent and direction of these changes, which in turn reflect a patient's current immune response state. As such, the new assay developed by SMART researchers is able to accurately and quickly assess patients' immune response states by profiling immune cell size, deformability, distribution, and cell counts.
Significantly, the new assay provides considerable advantages over existing methods of profiling the immune system and its activity. These include measuring leukocyte gene expression, cell-surface biochemical markers, and blood serum cytokine profile. Notably, these current methods require sample dilution or pre-processing steps, as well as labour-intensive, expensive equipment and antibody labelling procedures. As a result, these methods generally require a few hours, at minimum, to return results. This is a key pain point and drawback in triage and the emergency department, where clinicians need to make accurate clinical assessments as early as possible. The labour- and time-intensive nature of these current methods significantly limits their clinical utility for rapid triage and prevents their wider implementation within the ER or ICU.
In contrast, as this new SMART assay takes only 15 minutes, uses only 20 μL of whole blood, and only requires video capture frame rates of up to 150 fps, there is considerable potential for the technology to be developed into a portable unit that can perform point-of-care blood-sparing assays which could significantly improve the diagnosis and differentiation of patients in the ER and other primary or critical care settings. This application will enable clinicians to be able to quickly identify at-risk patients and take immediate action to mitigate or prevent organ dysfunction and other adverse effects of a hyper-aggressive immune response.
Lead author Dr Kerwin Kwek said, "Our new DLD assay will help address an unmet need in the ER and ICU by significantly reducing waiting time for accurate patient assay results. This could lead to more effective triage decision-making and more appropriate and timely treatment, which are critical to saving lives. More generally, this groundbreaking technology provides new insights into both the engineering of precision microfluidics and clinical research."
Professor Jongyoon Han added, "In the wake of lessons learnt in emergency rooms in hospitals across the world especially during the COVID-19 pandemic, where medical professionals have been faced with making difficult and at times life-or-death decisions in triage, this new technology represents a hugely exciting and significant breakthrough. By reducing the time taken for assay results from hours to a matter of minutes, SMART CAMP's new assay could help save lives as we continue to combat the scourge of pathogens and infectious diseases. The assay will also have wider applications, giving clinicians a new and more effective tool in the ER and ICU."
INFORMATION:
The research is carried out by SMART and supported by the National Research Foundation (NRF) Singapore under its Campus for Research Excellence And Technological Enterprise (CREATE) programme.
About Critical Analytics for Manufacturing Personalized-Medicine (CAMP)
CAMP is a SMART interdisciplinary research group launched in June 2019. It focuses on better ways to produce living cells as medicine, or cellular therapies, to provide more patients access to promising and approved therapies. The investigators at CAMP address two key bottlenecks facing the production of a range of potential cell therapies: critical quality attributes (CQA) and process analytic technologies (PAT). Leveraging deep collaborations within Singapore and MIT in the United States, CAMP invents and demonstrates CQA/PAT capabilities from stem to immune cells. Its work addresses ailments ranging from cancer to tissue degeneration, targeting adherent and suspended cells, with and without genetic engineering.
CAMP is the R&D core of a comprehensive national effort on cell therapy manufacturing in Singapore.
For more information, please visit: https://camp.smart.mit.edu/
About Singapore-MIT Alliance for Research and Technology (SMART)
Singapore-MIT Alliance for Research and Technology (SMART) is MIT's Research Enterprise in Singapore, established by the Massachusetts Institute of Technology (MIT) in partnership with the National Research Foundation of Singapore (NRF) since 2007. SMART is the first entity in the Campus for Research Excellence and Technological Enterprise (CREATE) developed by NRF. SMART serves as an intellectual and innovation hub for research interactions between MIT and Singapore. Cutting-edge research projects in areas of interest to both Singapore and MIT are undertaken at SMART. SMART currently comprises an Innovation Centre and five Interdisciplinary Research Groups (IRGs): Antimicrobial Resistance (AMR), Critical Analytics for Manufacturing Personalized-Medicine (CAMP), Disruptive & Sustainable Technologies for Agricultural Precision (DiSTAP), Future Urban Mobility (FM) and Low Energy Electronic Systems (LEES).
SMART research is funded by the National Research Foundation Singapore under the CREATE programme.
For more information, please visit: http://smart.mit.edu/
For media queries, please contact:
Glenn Tan
SMART@bluetotem.co
+65 9658 5749
For almost a century, physicists have been intrigued by the fundamental question: why are complex numbers so important in quantum mechanics, that is, numbers containing a component with the imaginary number i? Usually, it was assumed that they are only a mathematical trick to facilitate the description of phenomena, and only results expressed in real numbers have a physical meaning. However, a Polish-Chinese-Canadian team of researchers has proved that the imaginary part of quantum mechanics can be observed in action in the real world.
We need to significantly reconstruct our naive ...
Researchers at Lund University in Sweden have investigated how the X and Y chromosomes evolve and adapt to each other within a population. The results show that breaking up coevolved sets of sex chromosomes could lead to lower survival rates among the offspring - something that could be of importance in species conservation, for example. The study is published in the journal PNAS.
The results provide new clues on how species are formed, and suggest it could be harmful to bring together individuals from different populations that have been separated for a long time. The reason is that the offspring have lower survival rates.
"This is something worth keeping in mind in conservation biology, where you want to see a population ...
By clearing forests, burning grasslands, plowing fields and harvesting crops, humans apply strong selective pressures on the plants that survive on the landscapes we use. Plants that evolved traits for long-distance seed-dispersal, including rapid annual growth, a lack of toxins and large seed generations, were more likely to survive on these dynamic anthropogenic landscapes. In the current article, researchers argue that these traits may have evolved as adaptations for megafaunal mutualisms, later allowing those plants to prosper among increasingly sedentary human populations.
The new study hypothesizes that the presence of specific anthropophilic traits explains why a select few plant families came to dominate the crop and weed ...
The claim that old-growth forests play a significant role in climate mitigation, based upon the argument that even the oldest forests keep sucking CO2 out of the atmosphere, is being refuted by researchers at the University of Copenhagen. The researchers document that this argument is based upon incorrectly analysed data and that the climate mitigation effect of old and unmanaged forests has been greatly overestimated. Nevertheless, they reassert the importance of old-growth forest for biodiversity.
Old and unmanaged forest has become the subject of much debate in ...
One big challenge for the production of synthetic cells is that they must be able to divide to have offspring. In the journal Angewandte Chemie, a team from Heidelberg has now introduced a reproducible division mechanism for synthetic vesicles. It is based on osmosis and can be controlled by an enzymatic reaction or light.
Organisms cannot simply emerge from inanimate material ("abiogenesis"), cells always come from pre-existing cells. The prospect of synthetic cells newly built from the ground up is shifting this paradigm. However, one obstacle on this path is the question of controlled division--a requirement for having "progeny".
A team from the Max Planck Institute for Medical Research in Heidelberg, Heidelberg ...
The team of researchers at Kaunas University of Technology (KTU), Lithuania applied artificial intelligence (AI) methods to evaluate data of human embryo development. The AI-based system photographs the embryos every five minutes, processes the data of their development and notifies any anomalies observed. This increases the likelihood of choosing the most viable and healthy early-stage embryo for IVF procedures. The innovation was developed in collaboration with Esco Medical Technologies, a manufacturer of medical equipment.
Almost one in six couples face infertility; about 48.5 million couples, 186 million individuals worldwide are inflicted. Europe has one of the lowest birth rates in the world, with an average of just 1.55 children per woman.
The most effective form of ...
By offering a microscopic "tightrope" to cells, Virginia Tech and Johns Hopkins University researchers have brought new insights to the way migrating cells interact in the body. The researchers changed their testing environment for observing cell-cell interaction to more closely mirror the body, resulting in new observations of cells interacting like cars on a highway -- pairing up, speeding up, and passing one another.
Understanding the ways migrating cells react to one another is essential to predicting how cells change and evolve and how they react in applications, such as wound healing and drug delivery. In a study published in the Proceedings of the National Academy of Sciences, a team formed by Mechanical Engineering Associate ...
Scientists of Tomsk Polytechnic University jointly with colleagues from different countries have developed a new sensor with two layers of nanopores. In the conducted experiments, this sensor showed its efficiency as a sensor for one of the doping substances from chiral molecules. The research findings are published in Biosensors and Bioelectronics (IF: 10,257; Q1) academic journal.
The material is a thick wafer with pores of 20-30 nm in diameter. The scientists grew a layer of metal-organic frameworks (MOF) from Zn ions and organic molecules on these thick wafers. The MOF has about 3 nm nanopores only. It plays the role of a trap for molecules, which must be detected.
"This sensor can operate with chiral molecules. Such substances consisting of chiral molecules are a lot among medical ...
In the future, photovoltaic cells could be "worn" over clothes, placed on cars or even on beach umbrellas. These are just some of the possible developments from a study published in Nature Communications by researchers at the Physics Department of the Politecnico di Milano, working with colleagues at the University of Erlangen-Nuremberg and Imperial College London.
The research includes among its authors the Institute of Photonics and Nanotechnology (IFN-CNR) researcher Franco V. A. Camargo and Professor Giulio Cerullo. It focused on photovoltaic cells made using flexible organic technology. Today's most popular photovoltaic cells, based on silicone technology, are rigid and ...
The Miscanthus genus of grasses, commonly used to add movement and texture to gardens, could quickly become the first choice for biofuel production. A new study shows these grasses can be grown in lower agricultural grade conditions - such as marginal land - due to their remarkable resilience and photosynthetic capacity at low temperatures.
Miscanthus is a promising biofuel thanks to its high biomass yield and low input requirements, which means it can adapt to a wide range of climate zones and land types. It is seen as a viable commercial option for farmers but yields ...