NEW YORK, NY (AUGUST 26, 2024) — A team of scientists from The New York Stem Cell Foundation (NYSCF) Research Institute and Case Western Reserve University has created the largest reported collection of stem cell models from multiple sclerosis (MS) patients and used them to identify unique ways in which glia – integral support cells in the brain – contribute to the disease.
The study, published today in Cell Stem Cell, is the first to report that glial cells from MS patients have intrinsic hallmarks of disease, independent of immune system influences, which points to the power of stem cells for revealing new disease biology and to the need for new types of MS therapies.
The Hidden Roles of Glia in MS
MS is an autoimmune disease that occurs when the body’s immune system mistakenly attacks the protective myelin sheaths that surround the nerves in the brain and spinal cord, resulting in significant neurological disability.
“Most research and therapeutic strategies have so far focused on blocking the overactive immune system, but how cells in the brain itself, especially glia, contribute to the initiation and progression of MS remained a mystery,” explained Valentina Fossati, PhD, NYSCF Senior Research Investigator who led the study.
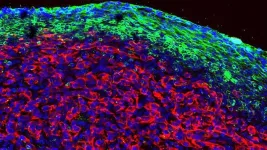
The team leveraged the power of NYSCF’s automation platforms to create induced pluripotent stem cells (iPSC) from skin biopsies of individuals with MS, resulting in the largest collection of MS patient stem cell lines to date, spanning diverse clinical subtypes. They then converted the iPSCs into glial cells – which include oligodendrocytes and astrocytes – to investigate their role in the disease.
“By generating glia-enriched cultures from stem cells, we have been able to study their role in MS independently of the complex environment in the body, which is constantly altered by the presence of immune cells and inflammatory signals,” continued Dr. Fossati.
Using single-cell gene expression profiling, the scientists found that stem cell-derived glia cultures from people with primary progressive MS (a particularly severe form of the disease) contained fewer oligodendrocytes. Oligodendrocytes are responsible for producing myelin, the protective sheath around nerve fibers that is lost in MS.
“This observation challenges the conventional understanding of MS as being purely driven by immune system dysfunction, suggesting that the disease may also be fueled by processes originating within the brain itself,” noted Paul Tesar, PhD, the Dr. Donald and Ruth Weber Goodman Professor of Innovative Therapeutics and director of the Institute for Glial Sciences at Case Western Reserve University School of Medicine, and NYSCF – Robertson Stem Cell Investigator Alumnus, who co-led the study.
The team also observed that a set of genes associated with immune and inflammatory functions were particularly active in stem cell-derived glia cultures from MS patients, matching what they see in brain samples from deceased individuals with MS. Moreover, NYSCF scientists leveraged their latest advances in artificial intelligence to detect differences in astrocytes that are not easily seen by the human eye.
“The fact that glia created from stem cells show similar features to glia in MS patient brains shows us that stem cell models provide a pretty accurate reflection of what happens in the brains of living patients, and that we can use them to gain important insights into this disease,” added Dr. Fossati.
A New Target for Therapeutic Intervention
Because of the autoimmune activity in MS, many current therapies target the immune system. These drugs help reduce the frequency of immune attacks, but they unfortunately fall short at preventing the neurodegeneration that drives disease progression.
The study’s findings open up new possibilities for treating MS. By identifying specific glial cell behaviors that contribute to the disease, researchers can now explore potential therapies that target these cells directly. This could lead to more effective treatments that go beyond simply suppressing the immune system and offer new hope for patients.
“Our findings represent a significant leap forward in our understanding of MS and underscore the vast potential in glia as a target for therapeutic intervention that could transform the treatment landscape for many patients,” remarked Dr. Tesar.
Breakthroughs Enabled by Team Science
This study was made possible by the powerful combination of NYSCF’s unique capabilities for large-scale stem cell-based disease modeling and the Tesar lab’s expertise in studying glia in neurological disease and developing new medicines. Patients were recruited at the Corinne Goldsmith Dickinson Center for Multiple Sclerosis through a collaboration with Ilana Katz Sand, MD, Associate Director of the CGD Center for MS, and Patrizia Casaccia, MD, PhD, now Director of the Neuroscience Initiative of the Advanced Science Research Center at the CUNY Graduate Center.
“This study is a remarkable example of team science,” said Jennifer J. Raab, NYSCF’s President and CEO. “It is through unique collaborations like these that we can move even faster towards new treatments for the major diseases of our time that patients urgently need.”
The experiments were led by co-first authors Benjamin Clayton, PhD, a National Multiple Sclerosis Society career transition fellow and newly appointed Assistant Professor at Case Western Reserve School of Medicine, and Lilianne Barbar, a former trainee in the Tesar and Fossati labs who is now an MD/PhD student at Washington University in St. Louis.
This work was supported by grants from the Department of Defense Congressionally Directed Medical Research Programs (CDMRP) Multiple Sclerosis Research Program (MSRP), award number DOD W81xWH-15-1-0448 (P.C.); the New York State Stem Cell Science (NYSTEM), award number C32586GG (V.F.); the National Multiple Sclerosis Society Career Transition Award TA-2105-37619 (B.L.L.C.); the National Institutes of Health, R35NS116842 (P.J.T.), R35NS111604 (P.C.), and sTF5 Care (P.J.T.); and by the New York Stem Cell Foundation Research Institute.
Full citation: Benjamin L.L. Clayton, Lilianne Barbar, Maria Sapar, Kriti Kalpana, Chandrika Rao, Bianca Migliori, Tomasz Rusielewicz, The NYSCF Global Stem Cell ArrayⓇ Team, Daniel Paull, Katie Brenner, Dorota Moroziewicz, Ilana Katz Sand, Patrizia Casaccia, Paul J. Tesar,* and Valentina Fossati*. Patient iPSC models reveal glia-intrinsic phenotypes in multiple sclerosis. Cell Stem Cell (2024). https://doi.org/10.1016/j.stem.2024.08.002
About The New York Stem Cell Foundation Research Institute
The New York Stem Cell Foundation (NYSCF) Research Institute is an independent non-profit organization accelerating cures and better treatments for patients through stem cell research. The NYSCF global community includes over 200 researchers at leading institutions worldwide, including the NYSCF – Druckenmiller Fellows, the NYSCF – Robertson Investigators, the NYSCF – Robertson Stem Cell Prize Recipients, and NYSCF Research Institute scientists and engineers. The NYSCF Research Institute is an acknowledged world leader in stem cell research and in the development of pioneering stem cell technologies, including the NYSCF Global Stem Cell Array®, which is used to create cell lines for laboratories around the globe. NYSCF focuses on translational research in an accelerator model designed to overcome barriers that slow discovery and replace silos with collaboration. For more information, visit nyscf.org.
About Case Western Reserve University
Case Western Reserve University is one of the country's leading private research institutions. Located in Cleveland, we offer a unique combination of forward-thinking educational opportunities in an inspiring cultural setting. Our leading-edge faculty engage in teaching and research in a collaborative, hands-on environment. Our nationally recognized programs include arts and sciences, dental medicine, engineering, law, management, medicine, nursing and social work. About 6,000 undergraduate and 6,300 graduate students comprise our student body. Visit case.edu to see how Case Western Reserve thinks beyond the possible.
END