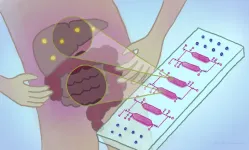
TAR-200 is a drug-device combination product that is inserted into the bladder and provides continuous, low-dose, local delivery of chemotherapy. "Our preliminary clinical trial found that TAR-200 was generally safe, well tolerated, and had beneficial effects on bladder cancer outcomes, in a group of patients with limited treatment options," comments lead author Mark Tyson, MD, MPH, of Mayo Clinic Arizona, Phoenix.
'Critical need' for new treatment options for MIBC
Bladder cancer is a common disease in older adults. By the time of diagnosis, about one-fourth of bladder cancers have spread into the muscle layer of the bladder wall. For these muscle-invasive bladder cancers (MIBCs), chemotherapy followed by surgery is the standard treatment.
However, some patients are considered medically unfit or decline to pursue this treatment, which carries substantial rates of complications and adverse effects. Based on previous studies, 25% to 57% of patients worldwide may not receive any "curative-intent" therapy for MIBC. "This suggests a critical need for alternative therapies that are tolerable and effective in an elderly population," the researchers write.
The TAR-200 is a small device implanted into the bladder, where it releases a continuous, low dose of gemcitabine, a standard chemotherapy agent. The goal of treatment is to limit cancer growth or progression while limiting the toxic effects of chemotherapy.
In the new phase one study, TAR-200 was used in 35 patients with MIBC: 24 men and 11 women, median age 84 years. The patients were deemed medically ineligible for standard surgery (radical cystectomy) and chemotherapy, or opted not to receive this treatment. All patients underwent minimally invasive surgery (transurethral resection of bladder tumor, or TURBT) to remove visible tumor.
The patients then underwent a simple procedure to place the TAR-200 device, which released gemcitabine over 21 days. At that time, another procedure was performed to remove and replace the device, for a total of four treatments over 84 days.
Few adverse events, evidence of efficacy with TAR-200
TAR-200 treatment was safe and well-tolerated, assessments suggested. About one-fourth of patients had problems related to device placement or treatment procedures. About 40% had some kind of treatment-related adverse event, most commonly related to problems with urination. These relatively minor problems were "as expected" in a group of frail elderly patients with MIBC, according to the authors. Just two patients were considered "not tolerant" of TAR-200, requiring device removal.
Overall, 11 of 35 patients had a complete tumor response to TAR-200, with no evidence of bladder cancer at follow-up. Three more patients had a partial response, for an overall response rate of 40%. Median overall survival was about 27 months. That compared to a 12 overall survival rate in previous studies of MIBC patients not receiving curative-intent treatment.
Among 14 patients with lasting responses to TAR-200 treatment, 70.5% remained free from progressive bladder cancer at 12 months after treatment. "Overall, the observed clinical response to TAR-200 was robust and durable in a cohort with very limited curative-intent treatment options," Dr. Tyson and coauthors conclude.
The researchers note the small size of their study, the lack of a comparison group, and incomplete assessment of response rates. Dr. Tyson comments, "Despite these limitations, the safety, patient tolerance, and promising preliminary effects of TAR-200 warrant further study as an alternative treatment for MIBC."
Read [Safety, Tolerability, and Preliminary Efficacy of TAR-200 in Patients With Muscle-invasive Bladder Cancer Who Refused or Were Unfit for Curative-intent Therapy: A Phase 1 Study]
Wolters Kluwer provides trusted clinical technology and evidence-based solutions that engage clinicians, patients, researchers and students in effective decision-making and outcomes across healthcare. We support clinical effectiveness, learning and research, clinical surveillance and compliance, as well as data solutions. For more information about our solutions, visit https://www.wolterskluwer.com/en/health and follow us on LinkedIn and Twitter @WKHealth.
###
About The Journal of Urology®
The Official Journal of the American Urological Association (AUA), and the most widely read and highly cited journal in the field, The Journal of Urology® brings solid coverage of the clinically relevant content needed to stay at the forefront of the dynamic field of urology. This premier journal presents investigative studies on critical areas of research and practice, survey articles providing brief editorial comments on the best and most important urology literature worldwide and practice-oriented reports on significant clinical observations. The Journal of Urology® covers the wide scope of urology, including pediatric urology, urologic cancers, renal transplantation, male infertility, urinary tract stones, female urology and neurourology.
About the American Urological Association
Founded in 1902 and headquartered near Baltimore, Maryland, the American Urological Association is a leading advocate for the specialty of urology, and has more than 23,000 members throughout the world. The AUA is a premier urologic association, providing invaluable support to the urologic community as it pursues its mission of fostering the highest standards of urologic care through education, research and the formulation of health care policy. To learn more about the AUA visit: www.auanet.org
About Wolters Kluwer
Wolters Kluwer (EURONEXT: WKL) is a global leader in professional information, software solutions, and services for the healthcare, tax and accounting, financial and corporate compliance, legal and regulatory, and corporate performance and ESG sectors. We help our customers make critical decisions every day by providing expert solutions that combine deep domain knowledge with specialized technology and services.
Wolters Kluwer reported 2022 annual revenues of €5.5 billion. The group serves customers in over 180 countries, maintains operations in over 40 countries, and employs approximately 20,000 people worldwide. The company is headquartered in Alphen aan den Rijn, the Netherlands.
For more information, visit www.wolterskluwer.com, follow us on LinkedIn, Twitter, Facebook, and YouTube.
END